Table of Contents
Overview
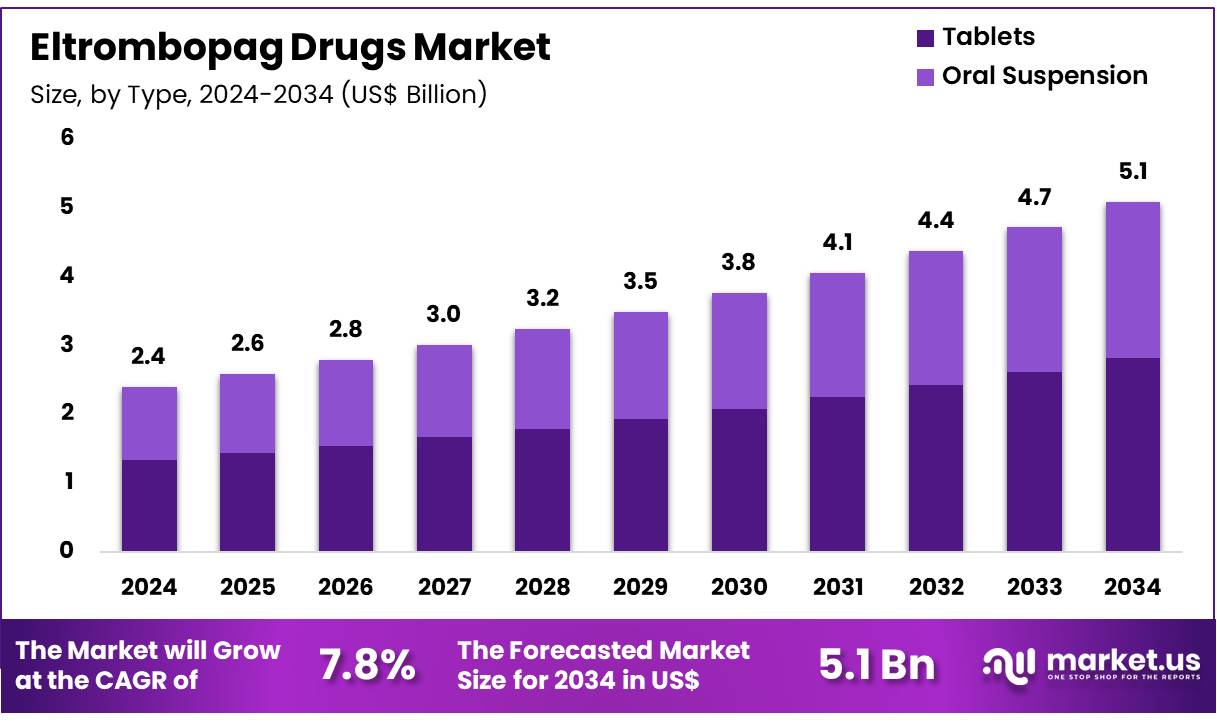
New York, NY – Dec 05, 2025 – Global Eltrombopag Drugs Market size is expected to be worth around US$ 5.1 Billion by 2034 from US$ 2.4 Billion in 2024, growing at a CAGR of 7.8% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 37.2% share with a revenue of US$ 0.9 Billion.
The introduction of Eltrombopag drugs has been positioned as a significant development within the therapeutic landscape for conditions associated with thrombocytopenia. Eltrombopag, an oral thrombopoietin receptor agonist, has been formulated to stimulate platelet production and support patients for whom conventional treatments have demonstrated limited efficacy. The drug has been developed to address chronic immune thrombocytopenia (ITP), severe aplastic anemia (SAA), and thrombocytopenia associated with chronic hepatitis C, where platelet insufficiency often restricts optimal disease management.
The growth of the Eltrombopag drug market has been attributed to the rising prevalence of platelet-related disorders and the increasing adoption of targeted therapies. The formulation of Eltrombopag has been designed to ensure consistent bioavailability, effective absorption, and therapeutic stability, thereby enabling improved clinical outcomes across diverse patient groups. Regulatory approvals across major markets have strengthened confidence in its safety and efficacy profile.
Market demand has been supported by enhanced healthcare awareness, improved diagnostic capabilities, and expanding treatment access in emerging economies. Pharmaceutical manufacturers have focused on scalable production strategies to meet global requirements, while ongoing research is expected to broaden therapeutic applications further. Strategic collaborations, product line extensions, and continued investment in clinical studies are anticipated to reinforce the drug’s competitive positioning.
Eltrombopag drugs are expected to maintain steady market growth as the global healthcare industry prioritizes advanced hematology treatments and patient-centric therapeutic solutions.
Key Takeaways
- In 2024, the global market for Eltrombopag drugs generated revenue amounting to US$ 2.4 billion. The market has been expanding at a CAGR of 7.8%, and revenue is projected to reach US$ 5.1 billion by 2034, supported by growing demand for advanced hematology therapeutics.
- The market, when segmented by type, is classified into tablets and oral suspension. Tablets accounted for the dominant position in 2024, capturing 55.3% of the total market share, driven by higher patient preference, ease of administration, and wider availability across healthcare settings.
- Based on application, the market is divided into hospitals & clinics, pharmacy, and other end users. The hospitals & clinics segment held the leading share of 60.8% in 2024, as most patients receive diagnosis, treatment initiation, and monitoring within these facilities.
- Regionally, North America emerged as the leading market in 2024, securing a 37.2% share. The region’s dominance can be attributed to strong healthcare infrastructure, higher diagnosis rates of thrombocytopenia-related disorders, and early adoption of advanced therapeutic solutions.
Regional Analysis
North America Leading the Eltrombopag Drugs Market
North America dominated the Eltrombopag drugs market in 2024, securing the highest revenue share of 37.2%. The strong regional performance has been attributed to the increasing diagnosis of chronic immune thrombocytopenia (ITP), severe aplastic anemia, and related hematological conditions for which Eltrombopag is an established therapeutic option. The region’s leadership is further supported by consistent product performance; Novartis reported Promacta/Revolade (Eltrombopag) sales of US$ 2,088 million in 2022 and US$ 2,216 million in 2023, reflecting steady demand and sustained clinical relevance.
Eltrombopag continues to play a critical role in managing thrombocytopenia among patients who exhibit insufficient response to conventional treatments. Regulatory oversight and ongoing approvals by the U.S. Food and Drug Administration (FDA) have ensured reliable access to the therapy, contributing to the stable expansion of the market across North America.
Asia Pacific Anticipated to Record the Highest CAGR
The Asia Pacific region is projected to witness the fastest CAGR during the forecast period. Growth in this region is expected to be driven by rising healthcare expenditures, heightened awareness of hematological disorders, and improving diagnostic infrastructure. According to World Bank 2022 data, current health expenditure in the East Asia & Pacific region stood at 6.6% of GDP, indicating sustained investment in healthcare systems and improved patient access to advanced therapeutics.
These structural advancements are anticipated to accelerate the adoption of Eltrombopag across Asia Pacific as diagnosis rates increase and treatment capabilities strengthen throughout emerging markets in the region.
Emerging Trends
- Broadening Clinical Indications: Eltrombopag, initially approved in 2008 for chronic immune thrombocytopenia, has progressively expanded into additional hematologic conditions. Regulatory authorizations for severe aplastic anemia in 2014 and hepatitis C–related thrombocytopenia in 2015 reflect continued validation of its thrombopoietic efficacy.
- Increasing Pediatric Utilization: The approval of eltrombopag olamine (ALVAIZ) in 2023 for children aged six years and above with persistent or chronic immune thrombocytopenia marks a pivotal advancement. This development signals growing confidence in its safety and effectiveness within pediatric hematology.
- Advancement in Combination Therapy Research: Several phase II clinical studies are examining eltrombopag in combination with therapies such as high-dose dexamethasone and cyclosporine. These regimens are being evaluated to improve response rates and extend therapeutic durability in immune thrombocytopenia and aplastic anemia cohorts.
- Evolving Regulatory Framework: The FDA’s updated draft guidance issued in October 2024 demonstrates evolving regulatory perspectives on eltrombopag olamine. The revisions address clinical trial design, safety monitoring parameters, and post-marketing requirements for thrombopoietin receptor agonists.
- Enhanced Pharmacokinetic Understanding: Recent updates indicate that a 75 mg/day dose of eltrombopag results in approximately a 3.2-fold higher area under the curve in patients with severe aplastic anemia compared with healthy individuals. These findings reinforce the importance of population-specific dosing strategies.
Frequently Asked Questions on Eltrombopag Drugs
- How does Eltrombopag work?
The drug functions by activating thrombopoietin receptors in bone marrow, resulting in improved platelet generation and reduced bleeding risk. The mechanism supports patients with inadequate platelet counts, and its targeted activity has strengthened its position in hematology treatment protocols globally. - What conditions are treated with Eltrombopag?
Eltrombopag is primarily used for immune thrombocytopenia, severe aplastic anemia, and hepatitis C–related thrombocytopenia. Its clinical utility has broadened as additional studies demonstrate improved hematologic response rates, thereby increasing physician confidence and patient access in multiple care settings. - What are the common side effects of Eltrombopag?
Typical adverse effects include nausea, fatigue, liver enzyme elevation, and headache. These effects are generally manageable under clinical supervision, and long-term safety observations indicate stable tolerability, supporting continued use across diverse patient demographics and treatment durations. - How is Eltrombopag administered?
Eltrombopag is administered orally once daily, usually on an empty stomach to improve absorption. Dosage is adjusted according to platelet response, and regular monitoring is required to maintain optimal therapeutic outcomes and minimize risks associated with elevated platelet levels. - Is Eltrombopag safe for long-term use?
Long-term use is considered safe when monitored appropriately, as clinical trials indicate sustained platelet response with manageable safety profiles. Regular liver function testing and dose adjustments ensure continued patient safety and effective disease management across extended treatment periods. - Which regions dominate the Eltrombopag market?
North America and Europe dominate due to advanced healthcare infrastructure, high diagnostic rates, and strong reimbursement support. Asia-Pacific is experiencing accelerated growth, driven by expanding patient populations, improving treatment awareness, and increasing investments in hematology therapeutics. - Who are the major market players?
Key participants include Novartis AG, generic manufacturers, and regional pharmaceutical companies. These firms focus on strategic collaborations, regulatory approvals, and product expansions to strengthen competitive positioning and capture growing demand across both developed and emerging markets.
Conclusion
The Eltrombopag drugs market has demonstrated consistent expansion, driven by rising thrombocytopenia prevalence, growing adoption of targeted hematology therapies, and broadening clinical indications. Strengthened regulatory approvals, increasing pediatric utilization, and expanding research initiatives continue to reinforce the drug’s therapeutic relevance.
Market growth remains supported by robust healthcare infrastructure in leading regions and rapidly improving diagnostic capabilities in emerging markets. With sustained investments in combination therapy development, scalable manufacturing, and population-specific dosing strategies, Eltrombopag is expected to maintain long-term clinical and commercial significance. The market outlook remains positive as global healthcare systems prioritize advanced, patient-centric hematology solutions.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



