Table of Contents
Overview
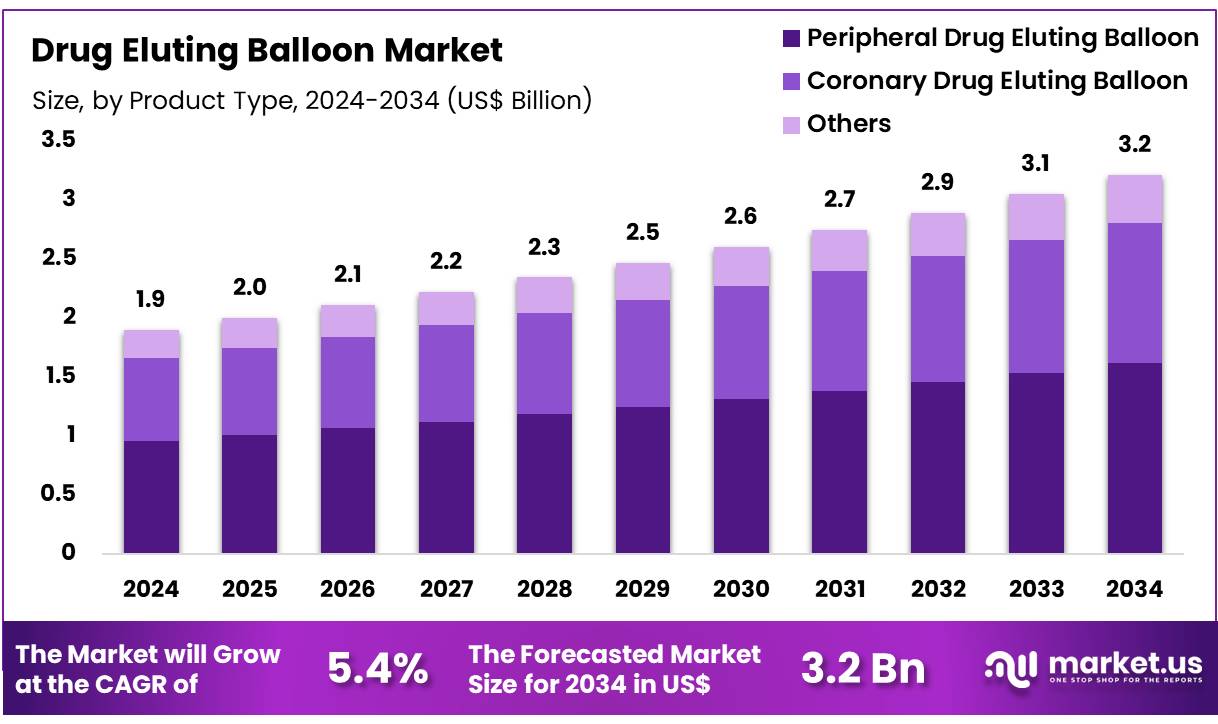
New York, NY – Dec 05, 2025 – Global Drug Eluting Balloon Market size is expected to be worth around US$ 3.2 Billion by 2034 from US$ 1.9 Billion in 2024, growing at a CAGR of 5.4% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 36.7% share with a revenue of US$ 0.7 Billion.
The Drug-Eluting Balloon (DEB) technology has been increasingly adopted across global healthcare systems, as rising demand for minimally invasive cardiovascular treatments continues to be observed. DEBs are catheter-based devices in which an angioplasty balloon is coated with an antiproliferative drug. The technology is designed to deliver a controlled therapeutic dose directly to the vessel wall during a short inflation period. The drug is rapidly absorbed, and the absence of a permanent implant allows natural vessel healing while reducing long-term complications.
The growth of the Drug-Eluting Balloon market has been attributed to the rising prevalence of coronary and peripheral artery diseases, an aging population base, and an expanding preference for non-stent treatment options. Increasing clinical evidence demonstrating improved patency rates, reduced restenosis, and lower repeat revascularization procedures has strengthened the adoption rate across hospitals and specialty clinics.
Product innovations, including advanced drug-coating formulations, improved balloon material, and enhanced drug transfer efficiency, have supported wider clinical acceptance. Regulatory approvals across North America, Europe, and Asia-Pacific have further contributed to faster commercialization and broader accessibility.
Investments in research and development continue to increase, as manufacturers focus on next-generation DEBs for complex lesions, small vessels, and in-stent restenosis. Strategic partnerships between medical device companies and healthcare institutions have also been observed, enabling technological advancements and expanded clinical application areas.
The Drug-Eluting Balloon market is expected to maintain steady growth, supported by ongoing improvements in interventional cardiology and strong emphasis on effective, implant-free treatment solutions.
Key Takeaways
- In 2024, the Drug Eluting Balloon market generated revenue of US$ 1.9 billion, and with a CAGR of 5.4%, the market is projected to reach US$ 3.2 billion by 2034.
- Based on product type, the market is categorized into peripheral drug eluting balloons, coronary drug eluting balloons, and others, with peripheral drug eluting balloons accounting for the leading share of 50.4% in 2024.
- In terms of technology, the market is segmented into Paccocath, Enduracoat, Freepac, Transpax, and others, wherein Paccocath represented the dominant share of 56.3%.
- Regarding end users, the market includes hospitals & ambulatory surgery centers, CATH laboratories, and others, with hospitals & ambulatory surgery centers holding the largest revenue share of 59.2%.
- Regionally, North America led the global market, securing a 36.7% share in 2024.
Regional Analysis
North America remained the leading region in the Drug Eluting Balloon market, accounting for the highest revenue share of 36.7%. The regional dominance has been supported by increasing regulatory approvals and the rising need for effective revascularization solutions in peripheral and coronary artery diseases. A key milestone was the U.S. FDA approval of Boston Scientific’s AGENT Drug-Coated Balloon in March 2024 for the treatment of coronary in-stent restenosis, representing the first approved coronary DEB in the United States and offering an important alternative to repeat stenting for patients with restenosis.
Market growth in North America has also been reinforced by strong performance from major industry players. Medtronic reported a 6.6% increase in revenue from its Cardiovascular Portfolio, reflecting sustained demand for interventional devices. Boston Scientific recorded full-year 2024 Peripheral Interventions net sales of US$2.410 billion, indicating reported growth of 14.2% and operational growth of 15.5% compared with 2023.
The Asia Pacific region is projected to register the fastest CAGR over the forecast period. Growth in the region is being driven by rising cardiovascular disease prevalence, rapid improvement in interventional cardiology infrastructure, and increasing healthcare expenditure. Regulatory momentum in China, with 6,247 imported medical devices approved in 2024, and expanded healthcare investment in India further support regional market expansion.
Emerging Trends
- Sirolimus-Coated Balloons: The introduction of sirolimus-coated drug-eluting balloons has been observed as an important advancement aimed at enhancing drug release kinetics and minimizing vessel inflammation. Early-stage clinical evaluations, including the SELUTION SLR™ trials, are being conducted to assess safety and therapeutic performance in both coronary and peripheral vascular applications.
- Expansion into Dialysis Access: The adoption of drug-coated balloons in the management of stenosis within arteriovenous dialysis fistulae has increased following the FDA approval of the IN.PACT AV DCB on November 21, 2019. Utilization in dialysis centers has expanded as clinicians integrate these devices into routine vascular access maintenance protocols.
- Heightened Safety Monitoring: Signals of elevated late mortality occurring approximately two to three years post-treatment with paclitaxel-coated devices have led regulatory authorities and clinicians to intensify long-term surveillance. Ongoing follow-up studies are being undertaken to clarify risk profiles and refine criteria for patient selection.
- Combination Therapies: Investigations are underway into therapeutic strategies that pair biomimetic stents with drug-eluting balloons for complex or recurrent arterial lesions. The combined approach seeks to integrate mechanical scaffolding with localized pharmacological intervention to improve treatment durability.
Use Cases
- Femoropopliteal Artery Disease: Evidence from a randomized clinical trial indicated that the Ranger™ DCB achieved a 12-month primary patency rate of 82.9%, compared with 66.3% for standard angioplasty. The improved outcome was associated with significant reductions in repeat revascularization.
- In-Stent Restenosis Prevention: The SurVeil™ DCB exhibited non-inferior outcomes against the IN.PACT Admiral® balloon, with both devices reporting a primary patency of 81.7% at one year. These findings support its applicability for treating in-stent restenosis.
- Dialysis Fistula Maintenance: The IN.PACT AV DCB has been indicated for percutaneous angioplasty of native arteriovenous fistulae up to 100 mm in lesion length and vessel diameters between 4 mm and 12 mm. The device provides a minimally invasive option intended to sustain long-term dialysis access function.
- Overall Patency Improvement: A recent meta-analysis reported that paclitaxel-coated balloons deliver an average 12-month vessel patency of 80.9%, compared with 57.5% achieved using uncoated balloons. This differential highlights their broad utility in the management of peripheral artery disease.
Frequently Asked Questions on Drug Eluting Balloon
- How does a drug-eluting balloon work?
The balloon is inflated for a short duration, allowing the drug coating to transfer into the vessel tissue. This localized drug delivery reduces neointimal hyperplasia, thereby lowering the likelihood of restenosis while preserving the vessel’s natural anatomy. - What drugs are commonly used on drug-eluting balloons?
Paclitaxel is the most widely used drug due to its lipophilic nature and rapid tissue uptake. Sirolimus-based drugs are increasingly being adopted, as their controlled release profile supports long-term vessel healing and reduces inflammation. - What are the advantages of drug-eluting balloons?
Their advantages include reduced restenosis, no requirement for permanent stent placement, and lower long-term complication rates. These benefits support improved vessel patency, shorter antiplatelet therapy durations, and greater flexibility for future treatment strategies. - Are drug-eluting balloons safe?
Clinical evidence indicates that drug-eluting balloons demonstrate favorable safety outcomes when used according to established guidelines. Their non-implant nature reduces long-term risks such as stent thrombosis while providing effective drug delivery to targeted lesions. - How do drug-eluting balloons differ from drug-eluting stents?
Drug-eluting balloons deliver therapeutic agents without leaving a scaffold, whereas drug-eluting stents provide structural support but remain permanently implanted. The absence of a foreign body in DEBs supports improved vessel flexibility and reduces chronic complications. - Which regions hold the largest market share?
North America and Europe currently dominate the market due to high adoption rates, advanced healthcare infrastructure, and strong clinical research activity. Asia-Pacific is emerging rapidly, supported by rising cardiovascular disease burden and expanding interventional cardiology capabilities. - What technological trends are shaping the market?
Innovations include next-generation sirolimus-coated balloons, improved polymer-free coatings, and enhanced drug-transfer technologies. These developments are aimed at achieving better therapeutic uniformity, longer drug retention, and improved outcomes across complex vascular segments. - Who are the key players in the drug-eluting balloon market?
Major companies include Medtronic, B. Braun, BIOTRONIK, BD, Koninklijke Philips, and Abbott. These firms have strengthened their market presence through regulatory approvals, product launches, and investments in advanced drug-coating and catheter delivery technologies.
Conclusion
The Drug-Eluting Balloon market is positioned for sustained expansion, supported by increasing cardiovascular disease prevalence, technological advancements, and growing clinical preference for implant-free interventions. Strong regulatory momentum, particularly in North America, and rapid healthcare infrastructure development in Asia-Pacific continue to accelerate global adoption.
Advancements in drug-coating technologies, rising use in complex lesions, and broader application across coronary, peripheral, and dialysis access segments further reinforce market growth. Ongoing clinical evidence demonstrating superior patency outcomes and reduced restenosis continues to validate therapeutic value. Overall, steady innovation and expanding clinical acceptance are expected to drive stable long-term market progression.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



