Table of Contents
Overview
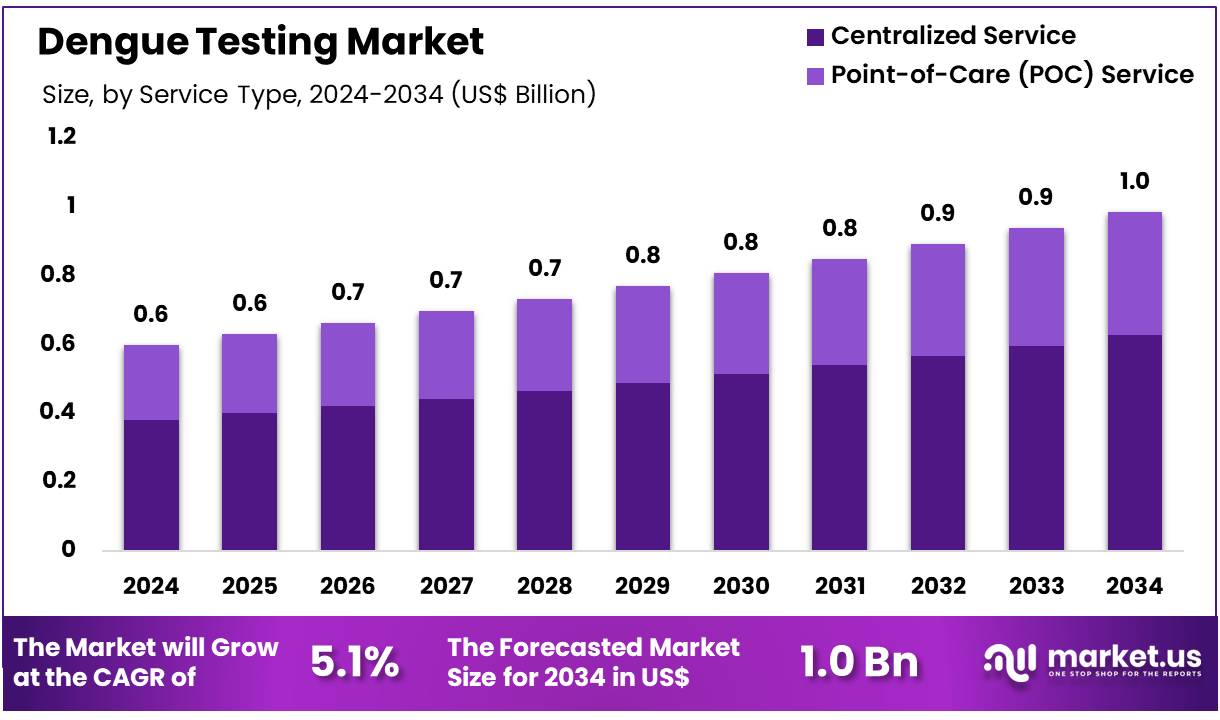
New York, NY – Dec 22, 2025 – Global Dengue Testing Market size is expected to be worth around US$ 1.0 Billion by 2034 from US$ 0.6 Billion in 2024, growing at a CAGR of 5.1% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 38.9% share with a revenue of US$ 0.2 Billion.
Dengue fever continues to be a significant public health concern in many tropical and subtropical regions. Early and accurate diagnosis plays a critical role in effective patient management and in reducing the risk of severe complications. Dengue testing is therefore considered an essential component of disease surveillance and clinical care.
Dengue testing is primarily used to detect infection caused by the dengue virus, which is transmitted through the bite of infected Aedes mosquitoes. The testing process helps confirm suspected cases, differentiate dengue from other febrile illnesses, and support timely medical intervention. Based on the stage of infection, different diagnostic methods are applied to ensure accuracy.
In the early phase of infection, laboratory tests focus on identifying viral components in the blood. These tests are effective within the first few days of symptom onset. As the infection progresses, antibody-based tests are commonly used to detect the immune response generated by the body against the virus. Both approaches are widely adopted in hospitals, diagnostic laboratories, and public health facilities.
Dengue testing is generally performed using a blood sample and is conducted under standardized laboratory conditions. The results assist healthcare professionals in determining disease severity, monitoring patient progress, and planning appropriate treatment strategies. Although there is no specific antiviral treatment for dengue, early diagnosis supports better clinical outcomes through proper supportive care.
With the rising incidence of dengue in many regions, the demand for reliable and accessible dengue testing is increasing. Strengthening diagnostic capacity is expected to support public health efforts aimed at disease control, outbreak management, and improved patient safety.
Key Takeaways
- In 2024, the dengue testing market generated revenue of approximately US$ 0.6 billion and is projected to expand at a CAGR of 5.1%, reaching an estimated value of US$ 1.0 billion by 2034.
- By product type, the market comprises ELISA-based tests, RT-PCR tests, rapid diagnostic tests, NS1 antigen detection kits, lateral flow immunoassays, and dengue IgG/IgM detection kits. Among these, ELISA-based tests emerged as the leading segment in 2023, accounting for a market share of 41.8%.
- On the basis of service type, the market is categorized into centralized services and point-of-care (POC) services. Centralized services dominated this segment, capturing a substantial share of 63.5%.
- In terms of end users, the dengue testing market is segmented into clinical laboratories, hospitals/clinics, and home healthcare settings. Clinical laboratories represented the largest segment, contributing 51.8% of the total market revenue.
- Regionally, North America maintained a leading position in the dengue testing market, holding a market share of 38.9% in 2024.
Regional Analysis
The dengue testing market in North America, representing 38.9% of the global market, experienced substantial growth in 2024, primarily driven by a significant increase in reported dengue cases and heightened public health initiatives. For instance, in Puerto Rico, a US territory, a public health emergency was declared in March 2024 due to rising dengue cases, with a total of 6,291 confirmed cases reported in 2024. This surge necessitated increased diagnostic testing to monitor the outbreak and manage patient care effectively.
The overall Region of the Americas, which includes North America, witnessed an alarming increase in dengue incidence, with over 7.6 million cases reported to WHO globally by April 30, 2024, surpassing the annual high of 4.6 million cases in 2023. This widespread increase across the Americas directly fueled the demand for accurate and rapid dengue diagnostics in North America.
Advancements in diagnostic technologies, such as the development of rapid NS1 antigen assays and multiplex PCR platforms, played a crucial role. These innovations provided faster and more reliable testing solutions, supporting early detection and improving patient outcomes, thereby driving market expansion. The continuous threat of imported cases due to increased global travel also spurred investments in laboratory upgrades and expanded point-of-care testing adoption, particularly in the US and Canada, bolstering the growth of the dengue testing sector.
The dengue testing market in Asia Pacific is anticipated to exhibit robust growth during the forecast period, propelled by the high endemicity of dengue, increasing urbanization, and expanding public health programs. Countries like India, Indonesia, and Thailand consistently report a substantial burden of dengue cases.
For example, India reported 289,235 dengue cases in 2023, the highest in the last 5 years, according to the National Centre for Vector Borne Diseases Control, underscoring the pressing need for extensive testing. Furthermore, as of August 2, 2024, India has already reported over 32,000 dengue cases, with 18,391 cases in the corresponding period of 2023, as stated by the Minister of State for Health and Family Welfare Prataprao Jadhav in the Lok Sabha. In addition, Karnataka reported 32,886 cases in 2024 (as of July 19, 2025 data), a significant increase from 19,300 cases in 2023.
Similarly, Malaysia, a part of the Western Pacific region, reported 17,497 cases in 2022, a 57.6% increase compared to the same period in the previous year, highlighting the escalating demand for diagnostics. Governments across the region are implementing national vector control programs, which frequently fund the deployment of ELISA and RT-PCR tests in regional laboratories and point-of-care NS1 rapid tests in community health centers.
Ongoing technological advancements, including the development of rapid diagnostic tests (RDTs) and molecular diagnostics, are expected to enhance testing accessibility and efficiency. Innovations like the introduction of portable RT-PCR molecular tests, such as one launched by Anitoa System in May 2022, and the development of high-accuracy dry luminescence assay tests, are further projected to drive market expansion by offering faster and more accurate diagnostic solutions in resource-constrained settings. These factors collectively indicate a strong growth trajectory for the dengue testing sector in Asia Pacific.
Emerging Trends
- Expansion of Point-of-Care (POC) Diagnostics: The adoption of portable and rapid diagnostic kits is increasing across community and field-based healthcare settings. These solutions support early dengue detection, minimize reliance on centralized laboratories, and enable faster initiation of treatment during outbreak situations.
- Rising Use of Multiplex Diagnostic Technologies: Multiplex platforms capable of simultaneously detecting dengue, Zika, Chikungunya, and other arboviruses are gaining traction. This trend is improving diagnostic efficiency, reducing testing time, and enhancing clinical accuracy in regions affected by multiple vector-borne diseases.
- Broader Integration of Molecular Diagnostic Methods: Affordable and accessible molecular diagnostics, particularly real-time RT-PCR, are being increasingly implemented. These techniques offer superior sensitivity during early infection stages and provide improved diagnostic accuracy compared to conventional serological testing methods.
- Adoption of Digital Health and Remote Reporting Systems: Mobile-enabled diagnostic devices and cloud-based data-sharing platforms are supporting faster result transmission. This integration enhances real-time disease surveillance, strengthens centralized data analysis, and enables more rapid and coordinated outbreak response.
- Development of Biomarker-Driven Prognostic Solutions: Advanced diagnostic tools focused on identifying biomarkers associated with dengue severity are under development. These innovations support early patient risk stratification and enable timely clinical interventions, contributing to reduced complication rates and improved patient survival outcomes.
Use Cases
- Disease Surveillance and Epidemiological Monitoring: Dengue testing is widely utilized by public health authorities for continuous surveillance activities. This enables precise outbreak mapping, timely containment measures, and optimized allocation of healthcare resources to high-risk regions.
- Early Detection in Endemic and High-Risk Areas: The deployment of rapid dengue diagnostics in primary and community healthcare facilities supports early disease identification. This facilitates prompt clinical management, reduces disease severity, and improves overall patient outcomes in endemic regions.
- Differential Diagnosis of Febrile Illnesses: Dengue testing plays a critical role in distinguishing dengue infections from other febrile illnesses such as malaria, leptospirosis, and typhoid. Accurate differentiation ensures appropriate treatment decisions and reduces the risk of clinical mismanagement.
- Screening of Travelers at International Entry Points: Testing of travelers arriving from dengue-endemic regions at airports, seaports, and border crossings supports early case identification. This approach helps limit cross-border transmission and strengthens global disease prevention strategies.
- Support for Clinical Research and Vaccine Development: In clinical trials and vaccine research programs, dengue diagnostics are essential for confirming infection status, monitoring participant outcomes, and evaluating vaccine efficacy. These applications support regulatory validation and future immunization initiatives.
Frequently Asked Questions on Dengue Testing
- What are the common types of dengue diagnostic tests available?
Common dengue diagnostic tests include NS1 antigen tests, IgM and IgG antibody tests, and molecular RT-PCR tests. Each method differs in sensitivity, detection window, cost, and suitability across different stages of infection. - When should dengue testing be performed after symptom onset?
Dengue testing is typically performed within the first five days of symptom onset using NS1 antigen or RT-PCR tests. After this period, antibody-based tests are preferred as immune response markers become detectable. - How accurate are dengue diagnostic tests?
The accuracy of dengue tests depends on the testing method, timing of sample collection, and laboratory quality. RT-PCR offers high sensitivity and specificity, while rapid diagnostic tests provide faster but moderately accurate results. - Can dengue testing differentiate between primary and secondary infection?
Yes, dengue testing can differentiate primary and secondary infections by analyzing IgM and IgG antibody levels. Secondary infections often show higher IgG titers, which is clinically significant due to increased severity risk. - What factors are driving growth in the dengue testing market?
Growth in the dengue testing market is driven by rising dengue incidence, expanding urban populations, climate change effects on mosquito vectors, and increasing awareness regarding early disease diagnosis and outbreak surveillance. - Which diagnostic segment dominates the dengue testing market?
The rapid diagnostic test segment dominates the dengue testing market due to low cost, quick turnaround time, and ease of use. These tests are widely adopted in resource-limited and high-burden dengue regions. - How does government involvement influence the dengue testing market?
Government initiatives significantly influence the dengue testing market through funding of public health programs, large-scale screening campaigns, and investments in laboratory infrastructure, particularly in dengue-endemic developing economies. - Which regions show the highest demand for dengue testing?
Asia-Pacific shows the highest demand for dengue testing due to high disease prevalence, dense population, and favorable climatic conditions. Latin America and parts of Africa are also experiencing rising demand driven by recurring outbreaks.
Conclusion
Dengue testing remains a critical pillar of global public health response, supporting early diagnosis, effective patient management, and outbreak control. The market growth is being driven by rising dengue incidence, expanding surveillance programs, and continuous advancements in diagnostic technologies. Widespread adoption of ELISA, RT-PCR, and rapid diagnostic tests has strengthened detection across both centralized laboratories and point-of-care settings.
Strong demand from endemic regions, particularly Asia Pacific, alongside growing preparedness in North America, highlights the expanding role of diagnostics. Overall, improved accessibility, accuracy, and integration of dengue testing are expected to enhance disease control and patient outcomes over the forecast period.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



