Table of Contents
Overview
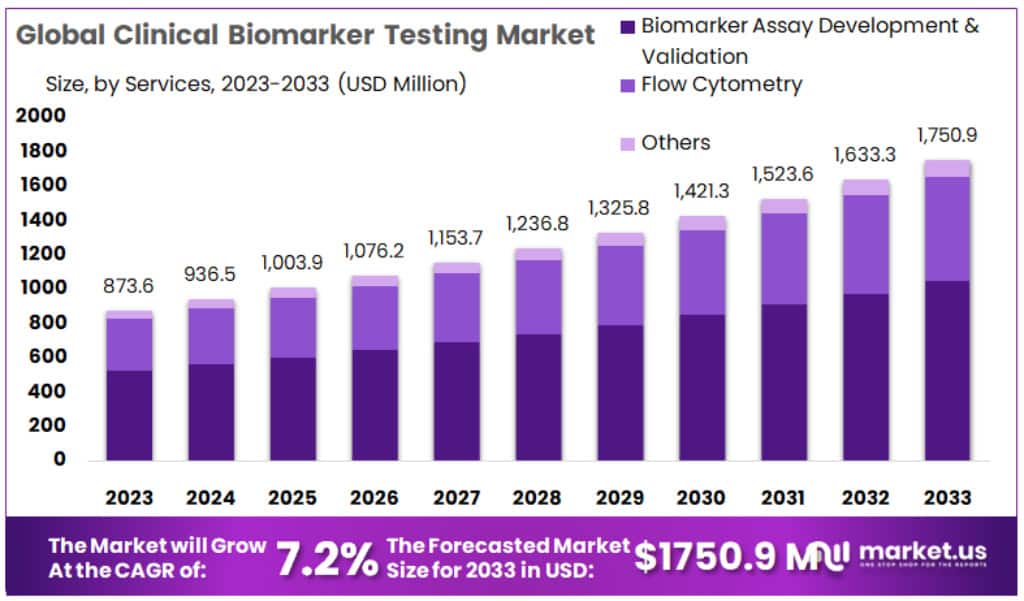
New York, NY – Jan 15, 2026 – The Global Clinical Biomarker Testing Market size is expected to be worth around USD 1750.9 Million by 2033 from USD 873.6 Million in 2023, growing at a CAGR of 7.2% during the forecast period from 2024 to 2033.
Clinical biomarker testing is a critical component of modern healthcare, supporting disease detection, diagnosis, prognosis, and treatment monitoring. Biomarkers are measurable biological indicators found in blood, tissue, or other bodily fluids, and they provide objective data on normal or pathological processes, as well as responses to therapeutic interventions.
The adoption of clinical biomarker testing has increased significantly due to its role in enabling precision medicine. By identifying specific molecular or genetic characteristics of a disease, biomarker testing allows treatments to be tailored to individual patients. This approach improves clinical outcomes, reduces trial-and-error prescribing, and enhances overall healthcare efficiency.
Biomarker tests are widely used across multiple therapeutic areas, including oncology, cardiology, neurology, and infectious diseases. In cancer care, biomarkers are used to identify tumor subtypes, predict treatment response, and monitor disease progression. In chronic and infectious conditions, they support early detection and ongoing disease management.
Technological advancements such as next-generation sequencing, immunoassays, and molecular diagnostics have strengthened the accuracy, speed, and reliability of biomarker testing. As a result, testing has become more accessible across hospitals, diagnostic laboratories, and research institutions.
The growth of clinical biomarker testing is driven by rising chronic disease prevalence, increasing demand for personalized therapies, and strong investment in life sciences research. Regulatory support and expanding clinical validation further contribute to market expansion.
Key Takeaways
- Market Size: The Clinical Biomarker Testing Market is projected to reach approximately USD 1,750.9 million by 2033, increasing from USD 873.6 million recorded in 2023.
- Market Growth: The market is anticipated to expand at a compound annual growth rate (CAGR) of 7.2% throughout the forecast period from 2024 to 2033.
- Services Analysis: Biomarker Assay Development and Validation emerged as the leading service segment, accounting for over 59.5% of the total market share in 2023.
- End-Use Analysis: Pharmaceutical and biotechnology companies represented the largest end-use segment, holding more than a 43.6% share of the market in 2023.
- Regional Analysis: North America continues to dominate the global market, capturing approximately 46% of total revenue, equivalent to USD 401.8 million in 2023.
- Technological Advancements: Ongoing progress in genomics, proteomics, and bioinformatics is significantly improving biomarker discovery and validation processes, resulting in enhanced test accuracy and operational efficiency.
- High Investment in R&D: Substantial investments in research and development are supporting the identification of novel biomarkers and the advancement of dependable and cost-efficient testing solutions.
- Partnerships and Collaborations: Strategic alliances among pharmaceutical companies, biotechnology firms, and diagnostic laboratories are playing a vital role in accelerating biomarker innovation and clinical adoption.
Regional Analysis
North America continues to dominate the global biomarker testing market, accounting for a substantial 46% share, equivalent to USD 401.8 million in 2023. This leadership position is largely driven by the strong presence of established market participants, advanced healthcare infrastructure, and high levels of awareness regarding the clinical value of biomarker-based diagnostics.
In addition, significant investments in research and development have supported continuous technological advancements. However, the region operates within a highly regulated framework. While these regulations enhance test accuracy, quality, and patient safety, they also increase compliance costs and may limit market entry for smaller or emerging companies.
Europe represents the second-largest regional market, supported by increasing adoption of biomarker testing, well-developed healthcare systems in Western Europe, and expanding public healthcare initiatives in Eastern Europe. Western European countries demonstrate higher market maturity due to early adoption and stronger reimbursement mechanisms, whereas Eastern Europe is experiencing accelerated growth. Despite this progress, the European market remains fragmented, primarily due to variations in reimbursement structures, regulatory processes, and healthcare policies across individual countries.
Asia Pacific is expected to witness the fastest growth during the forecast period, with a current market value of USD 156.6 million. Growth is supported by a large patient base, rising disposable incomes, and increasing incidence of chronic diseases. Countries such as China and India are playing a pivotal role through supportive government policies and expanding research activities. Nonetheless, disparities in healthcare access and shortages of skilled professionals continue to restrain growth in certain markets.
Emerging Trends
- Integration of Artificial Intelligence (AI): Artificial intelligence is being increasingly adopted for the analysis of complex biomarker datasets. Advanced AI algorithms enable the identification of subtle patterns and correlations that are often undetectable through conventional analytical techniques. As a result, improvements in diagnostic accuracy, disease stratification, and prognosis assessment are being achieved.
- Adoption of Multi-Omics Approaches: The integration of genomics, proteomics, and metabolomics data is enabling a more comprehensive understanding of disease biology. This multi-omics framework supports the identification of novel biomarkers and enhances the development of personalized and precision-based therapeutic strategies.
- Growth of Liquid Biopsy Technologies: Non-invasive liquid biopsy techniques, particularly those detecting circulating tumor DNA (ctDNA), are gaining significant momentum in oncology diagnostics. These approaches allow for mutation detection and treatment selection without the need for invasive tissue biopsies, improving patient comfort and accelerating clinical decision-making.
- Expansion of Real-World Data Analytics: The utilization of real-world data, including electronic health records and patient registries, is accelerating biomarker discovery and clinical validation. This data-driven approach supports more effective treatment development and contributes to improved clinical outcomes across multiple disease areas.
Use Cases
- Cancer Therapy Selection: Biomarker testing plays a critical role in identifying tumor-specific genetic alterations, thereby guiding the selection of targeted therapies. For instance, mutation profiling in oncology enables clinicians to match patients with therapies that demonstrate higher efficacy and reduced adverse effects.
- Cardiovascular Risk Evaluation: Inflammatory biomarkers, such as high-sensitivity C-reactive protein (hs-CRP), are widely used to assess cardiovascular risk. Elevated biomarker levels are associated with an increased likelihood of adverse cardiac events, supporting early intervention and preventive care strategies.
- Neurological Disease Diagnosis and Monitoring: Biomarkers including amyloid-beta and tau proteins are used to support the diagnosis and progression monitoring of neurodegenerative disorders. Their measurable presence in biological fluids provides valuable insights into disease onset and progression, facilitating earlier clinical intervention.
- Infectious Disease Management: Biomarker-based testing, such as procalcitonin measurement, is increasingly used to differentiate bacterial infections from viral conditions. This enables more appropriate antibiotic prescribing practices and supports global efforts to reduce antimicrobial resistance.
- Autoimmune Disease Assessment: In autoimmune disorders, including rheumatoid arthritis, disease-specific biomarkers are employed for diagnosis and ongoing disease monitoring. These biomarkers support timely treatment adjustments and improved long-term disease management.
Frequently Asked Questions on Clinical Biomarker Testing
- What are the main types of clinical biomarkers used in testing?
Clinical biomarker testing commonly includes diagnostic, prognostic, predictive, pharmacodynamic, and safety biomarkers, each serving distinct roles in identifying disease status, forecasting outcomes, monitoring therapy effectiveness, and assessing potential treatment-related risks in patients. - How is clinical biomarker testing used in disease management?
Clinical biomarker testing is used to support early disease detection, stratify patient populations, guide therapy selection, monitor treatment response, and assess disease recurrence, thereby improving clinical outcomes and reducing unnecessary or ineffective medical interventions. - What technologies are used in clinical biomarker testing?
Technologies used in clinical biomarker testing include immunoassays, polymerase chain reaction, next-generation sequencing, mass spectrometry, and bioinformatics platforms, which collectively enhance sensitivity, specificity, and throughput of biomarker detection and analysis. - What are the key benefits of clinical biomarker testing?
The primary benefits of clinical biomarker testing include improved diagnostic accuracy, personalized treatment planning, reduced adverse drug reactions, optimized healthcare costs, and enhanced clinical trial efficiency by identifying responsive patient subgroups more effectively. - What factors are driving growth in the clinical biomarker testing market?
Market growth is driven by increasing prevalence of chronic diseases, expanding oncology research, technological advancements in molecular diagnostics, rising adoption of personalized medicine, and growing investments in drug development and clinical research activities worldwide. - Which application areas dominate the clinical biomarker testing market?
Oncology represents the dominant application area, followed by cardiology, neurology, infectious diseases, and autoimmune disorders, as biomarker-based testing plays a critical role in cancer diagnosis, prognosis, and therapy selection across healthcare systems. - What is the future outlook of the clinical biomarker testing market?
The market outlook remains cautiously optimistic, supported by ongoing advances in genomics, proteomics, and artificial intelligence, alongside increasing clinical integration of biomarkers, which is expected to enhance precision medicine adoption and long-term market expansion.
Conclusion
Clinical biomarker testing has become an essential pillar of modern healthcare, enabling earlier diagnosis, precise disease stratification, and personalized treatment strategies across multiple therapeutic areas. Market growth is being driven by rising chronic disease prevalence, expanding oncology applications, and continuous advancements in genomics, proteomics, and data analytics.
Strong investment in research and development, along with strategic collaborations, is accelerating innovation and clinical adoption. While regulatory complexity and regional disparities remain challenges, the overall outlook remains cautiously optimistic. Continued technological progress and integration into routine clinical practice are expected to support sustained market expansion over the long term.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



