Table of Contents
Introduction
The Global Glioblastoma Multiforme Treatment Market size is expected to be worth around US$ 4.7 Billion by 2033, from US$ 2.2 Billion in 2023, growing at a CAGR of 8.1% during the forecast period from 2024 to 2033. In 2023, North America led the market, achieving over 42.3% share with a revenue of US$ 0.9 Billion.
This market growth is fueled by advancements in surgical techniques and pharmacotherapy. Surgeons now use intraoperative mapping and image-guided surgery, which improves the precision of tumor removal and minimizes damage to healthy brain tissue, leading to better patient outcomes. Post-surgery treatments, such as radiotherapy and temozolomide-based chemotherapy, are also becoming more targeted, with treatments tailored to patients’ genetic profiles, such as MGMT methylation status.
Emerging therapies like immunotherapy, including dendritic cell vaccines and checkpoint inhibitors, are showing promise in treating GBM. Additionally, the development of targeted therapies, based on the specific molecular characteristics of tumors, is shifting the focus toward personalized treatment options. Ongoing research and clinical trials are essential in discovering new therapies and improving existing ones.
Integrative treatment approaches, such as art therapy, meditation, and exercise, are also being used to enhance the quality of life for GBM patients during treatment.
Recent developments in the field include strategic partnerships and clinical trials. In October 2024, Teva and mAbxience expanded their partnership to include a new oncology biosimilar for GBM. In September 2024, Pfizer announced that its drug Crizotinib was in Phase II clinical trials, showing a 23% phase transition success rate. Additionally, in June 2024, Merck and DNAtrix launched a Phase 2 study to evaluate the combined effect of the oncolytic immunotherapy DNX-2401 and Merck’s KEYTRUDA for recurrent GBM, focusing on synergistic treatment strategies.
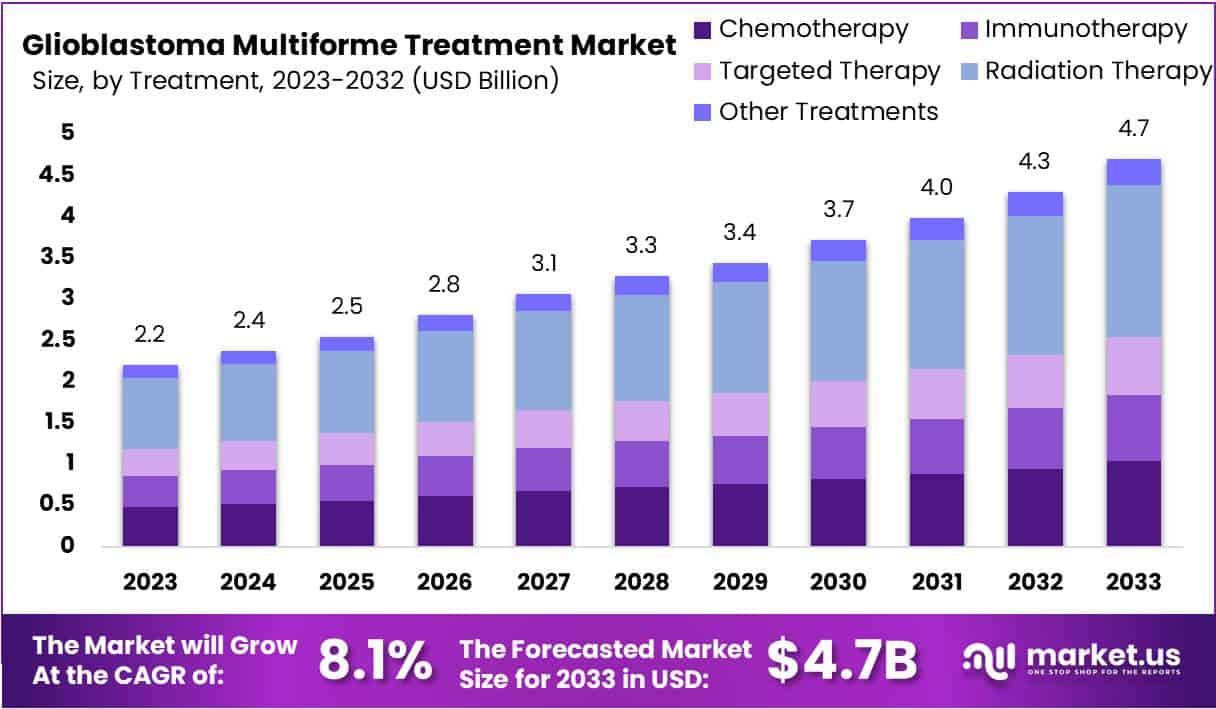
Key Takeaways
- The Glioblastoma Multiforme Treatment Market is set to grow by 8.1% annually, reaching USD 4.7 billion by 2033.
- Radiation Therapy, precise and sparing to healthy tissues, holds a 39.2% market share.
- Bevacizumab, leading in drug classes, captures a 29% share by effectively blocking angiogenesis.
- Hospitals, equipped with specialized capabilities, are the main treatment centers, with over 47% market dominance.
- Advances in targeted and immune therapies are pushing the market’s growth.
- Rising global cases of glioblastoma multiforme boost the demand for advanced treatments.
- Collaborative research efforts are accelerating clinical trials and innovations in treatment.
- Government support is pivotal in funding research and development for glioblastoma treatments.
- Challenges like low treatment success rates and stringent regulations hinder progress.
- Trends like Precision Medicine, AI integration, and combination therapies are carving new paths for treatment.
- In 2023, North America led the market, holding a 42.3% share, due to superior healthcare and research facilities.
Glioblastoma Multiforme (GBM) Treatment Statistics
- Definition: Glioblastoma multiforme (GBM) is a WHO grade IV glioma and the most aggressive primary brain tumor.
- Prevalence: GBM accounts for approximately 15.6% of all brain tumors and 45.2% of primary malignant brain tumors.
- Age and Diagnosis: GBM is most commonly diagnosed in individuals over the age of 65, with a median survival of about 12 to 15 months following diagnosis.
- Survival Rate: The overall 5-year survival rate for GBM patients is low, at just 7.2%.
- Standard Treatment: The typical treatment regimen begins with surgical resection, followed by chemoradiotherapy.
- Surgical Impact: Complete surgical resection significantly improves both progression-free survival (PFS) and overall survival (OS) compared to partial resection.
- Radiotherapy: The standard radiotherapy protocol involves 60 Gy in 2 Gy fractions over six weeks for patients under the age of 70.
- Radiotherapy for Older Adults: For patients over 70, hypofractionated short-course radiotherapy (40 Gy in 15 fractions over three weeks) is often used.
- Radiotherapy Outcomes: There is no significant survival difference between hypofractionated short-course and standard radiotherapy, with survival times of 5.6 months and 5.1 months, respectively.
- First-line Chemotherapy: Temozolomide (TMZ) is the standard chemotherapy, though its effectiveness varies depending on the levels of the MGMT protein in tumor cells.
- Advanced Chemotherapy: Adding Bevacizumab to lomustine increases the median progression-free survival (PFS) to 4.2 months, up from 1.5 months with lomustine alone.
- Combined Treatment: When Bevacizumab is combined with standard radiotherapy and TMZ, the median PFS increases to 10.6 months, compared to 6.2 months with a placebo.
- Localized Chemotherapy: Carmustine wafers (Gliadel) are implanted directly at the tumor site after resection, delivering localized chemotherapy.
- Clinical Trials: Ongoing trials are exploring the potential of immune checkpoint inhibitors like nivolumab and atezolizumab for treating GBM.
- Focused Ultrasound (FUS): Clinical trials are investigating the use of focused ultrasound to enhance drug delivery across the blood-brain barrier.
- Seizure Incidence: Around 20-50% of GBM patients experience seizures, often as an early symptom.
- Urinary Incontinence: About 40% of GBM patients report urinary incontinence, caused by tumor effects on the nervous system.
- Dysphagia Prevalence: Dysphagia, which affects 26-85% of GBM patients, complicates swallowing and increases health risks.
- Annual Incidence: GBM is diagnosed in 3-4 new cases per 100,000 people annually.
- DNA Repair Challenges: GBM’s strong DNA repair mechanisms contribute to its resistance to current treatments.
- Oxidative Stress: Increased reactive oxygen species (ROS) in GBM cells hinder DNA repair, contributing to tumor progression.
- DNA Damage Effects: ROS-induced DNA damage accelerates glioma cell proliferation and invasion.
- Prognostic Models: Bioinformatics is used to develop prognostic models that aid in early diagnosis and guide therapy development.
- Gene Expression Analysis: Identifying differentially expressed genes (DEGs) between GBM and normal brain tissue is crucial for targeted treatment strategies.
- Survival Statistics: In 2022, the average survival rate after biopsy was 6.6 months. An estimated 72,360 adults aged 40+ were diagnosed with a primary brain tumor in the U.S., with gliomas representing 81% of all malignant brain tumors.
Emerging Trends in Glioblastoma Treatment
- Advancements in CAR T-cell Therapies: The treatment of glioblastoma is progressing with the development of CAR T-cell therapies. These therapies are designed to target specific markers found on brain tumor cells, such as ROBO1. Early preclinical studies show that these advanced treatments have the potential to significantly extend patient survival, possibly doubling survival times. This trend represents a shift toward more precise and effective immunological treatments for glioblastoma.
- Development of Immunotherapy Vaccines: New immunotherapy vaccines are being developed to boost the immune system’s ability to fight glioblastoma. One such vaccine, the rWTC-MBTA, combines irradiated tumor cells with immune-stimulating compounds. This approach aims to build a strong immune response specifically targeted at glioblastoma cells, offering a promising strategy to enhance the body’s defense against the tumor.
- Ultrasound Enhanced Immunotherapy: Researchers are exploring the use of ultrasound technology to improve the delivery and effectiveness of immunotherapy. By modifying the tumor’s microenvironment using ultrasound waves, the tumors become more susceptible to immune system attacks. This technique aims to optimize the delivery of therapeutic agents to tumors, potentially improving treatment outcomes by making therapies more targeted and effective.
- Gamma Delta T Cell Therapy: Gamma delta T cell therapy is an innovative approach to glioblastoma treatment, utilizing genetically modified T cells in combination with temozolomide chemotherapy. Early results suggest that this therapy can improve progression-free survival and may outperform traditional treatment methods, offering new hope for patients with this aggressive cancer.
- Exploration of Small Molecule Inhibitors: Researchers are investigating small molecule inhibitors that target key molecular motors involved in tumor growth and resistance. These inhibitors aim to disrupt the mechanisms tumors use to evade treatments like radiation. By targeting these molecular pathways, small molecule therapies could significantly enhance the effectiveness of current glioblastoma treatments, potentially providing long-lasting therapeutic options.
Use Cases in Glioblastoma Treatment
- CAR T-cell Therapy Application: CAR T-cell therapy has shown promising results in treating recurrent glioblastoma. This treatment works by modifying a patient’s T-cells to recognize and attack cancer cells more effectively, leading to significant tumor reduction and extended progression-free survival for many patients. These promising results highlight the potential of CAR T-cell therapy in treating recurrent brain tumors.
- Vaccine Trials: The rWTC-MBTA vaccine has shown encouraging results in glioblastoma models, prolonging survival and enhancing the effectiveness of standard therapies. By stimulating both innate and adaptive immune responses, this vaccine could become an important part of glioblastoma treatment regimens. Its success in trials offers hope for incorporating vaccine therapy into standard treatment protocols, potentially revolutionizing the way glioblastoma is managed.
- Ultrasound in Immunotherapy Delivery: The use of ultrasound technology to enhance immunotherapy delivery is an innovative approach to treating glioblastoma. Early clinical applications have demonstrated that ultrasound can modify the tumor environment, improving the impact of immunotherapy treatments. This technique has the potential to improve treatment outcomes by making immunotherapy more effective and better targeted at the tumor.
- Gamma Delta T Cell Therapy Trials: Gamma-delta T cell therapy, when combined with standard treatments like temozolomide, has shown improved outcomes in clinical trials, especially in terms of progression-free survival. This therapy harnesses a potent subset of T cells that can directly attack tumor cells. The increased survival rates observed in these trials suggest that gamma-delta T cell therapy could serve as an effective complement to conventional glioblastoma treatments.
- Small Molecule Combination Therapy: Combining small molecule inhibitors with radiation therapy has proven effective in laboratory studies for overcoming tumor growth and resistance in glioblastoma. This combination strategy targets various pathways involved in tumor survival and proliferation, enhancing the effects of radiation. As these therapies advance in clinical trials, they have the potential to reshape standard care, offering new treatment options for glioblastoma patients.
Conclusion
The Glioblastoma Multiforme (GBM) treatment landscape is evolving with significant advancements in surgical techniques, targeted therapies, and immunotherapy. Precision medicine, including tailored treatments based on genetic profiles and emerging therapies like CAR T-cell therapy and immunotherapy vaccines, is driving progress in patient outcomes.
Collaborative research and ongoing clinical trials continue to explore innovative treatments, while integrative approaches are enhancing quality of life for patients. Despite challenges, such as low survival rates and resistance to treatment, these advancements offer hope for improved management and potential breakthroughs in the fight against GBM.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



