Table of Contents
Overview
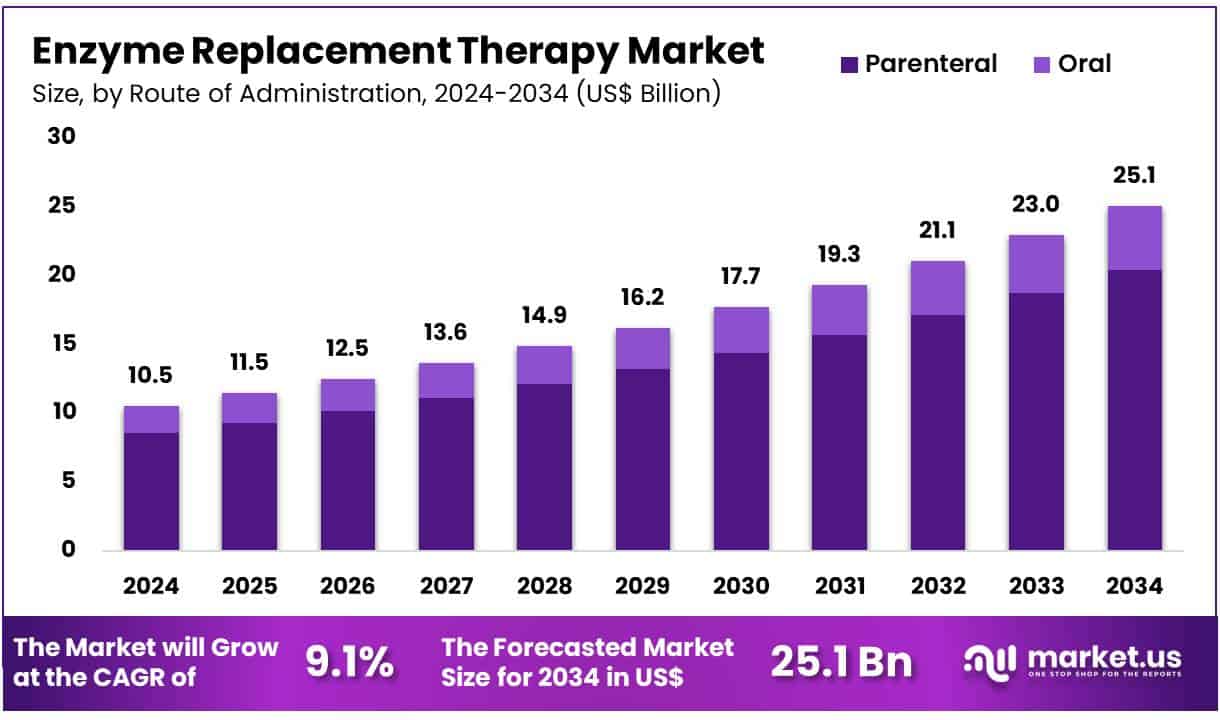
New York, NY – July 08, 2025 – Global Enzyme Replacement Therapy Market size is expected to be worth around US$ 25.1 Billion by 2034 from US$ 10.5 Billion in 2024, growing at a CAGR of 9.1% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 39.8% share with a revenue of US$ 4.2 Billion.
The global Enzyme Replacement Therapy (ERT) market is poised for sustained growth due to increasing prevalence of rare and inherited metabolic disorders, including Gaucher disease, Fabry disease, Pompe disease, and mucopolysaccharidoses (MPS). ERT is a medical treatment that involves replacing a deficient or absent enzyme in patients with such disorders, thereby improving cellular function and disease management.
Recent advancements in biotechnology and increased regulatory approvals have expanded the treatment landscape. Therapies developed using recombinant DNA technology have enabled targeted and safer delivery of enzymes, reducing long-term complications and improving patient outcomes. According to the U.S. National Institutes of Health (NIH), more than 7,000 rare diseases exist globally, many of which are linked to enzyme deficiencies, underlining the growing need for advanced therapeutic interventions.
North America currently leads the market due to a strong healthcare infrastructure, active clinical trials, and significant investments by key pharmaceutical players. However, the Asia-Pacific region is projected to witness the fastest growth, driven by increasing awareness, rising healthcare expenditure, and government support for orphan disease treatment.
Furthermore, ongoing research into novel delivery mechanisms, such as intrathecal and gene therapy-adjacent approaches, continues to drive innovation in ERT. The market outlook remains cautiously optimistic as healthcare systems prioritize treatments for rare and underserved patient populations.
Key Takeaways
- In 2024, the global Enzyme Replacement Therapy (ERT) market generated a revenue of US$ 10.5 billion and is projected to reach approximately US$ 25.1 billion by 2034, expanding at a compound annual growth rate (CAGR) of 9.1% during the forecast period.
- By product type, the market is segmented into imiglucerase, agalsidase beta, taliglucerase, velaglucerase alfa, and others. Among these, imiglucerase emerged as the leading product in 2023, accounting for 39.8% of the total market share.
- Based on the route of administration, the market is categorized into parenteral and oral. The parenteral route held the dominant position, contributing 52.3% of the market revenue in 2023.
- With respect to therapeutic condition, the market is segmented into Gaucher disease, Pompe disease, Severe Combined Immunodeficiency (SCID), Fabry disease, and others. Gaucher disease accounted for the largest share, representing 81.4% of the total market.
- In terms of end-user, the market is divided into infusion centers, hospitals, and others. Infusion centers led the segment in 2023, capturing a 45.6% revenue share.
- Regionally, North America dominated the global market, securing a 39.8% share in 2023, supported by advanced healthcare infrastructure and early adoption of biologic therapies
Segmentation Analysis
- Product Type Analysis: Imiglucerase continues to lead the enzyme replacement therapy market with a 39.8% share, primarily due to its proven efficacy in treating Gaucher disease. Its long-established clinical use and reliability have made it the preferred treatment. Continued demand is expected as patient awareness grows. Additionally, advancements in biosimilars and formulations are likely to enhance accessibility and affordability, reinforcing imiglucerase’s market dominance and expanding its adoption in long-term disease management.
- Route of Administration Analysis: Parenteral administration holds a dominant 81.4% share in the enzyme replacement therapy market, driven by its precision in delivering effective treatment for enzyme deficiencies. Intravenous and subcutaneous injections remain the standard for most ERTs due to their reliability and consistent outcomes. As clinical support for parenteral ERTs expands and infusion centers proliferate, adoption across hospital and outpatient settings is expected to rise, ensuring sustained preference for parenteral routes in enzyme replacement protocols.
- Therapeutic Condition Analysis: Gaucher disease accounts for 52.3% of the enzyme replacement therapy market, making it the largest therapeutic condition segment. This rare disorder, caused by glucocerebrosidase deficiency, requires lifelong ERT. Increased diagnosis rates, improved genetic screening, and the availability of effective therapies like imiglucerase are driving segment growth. Greater awareness and the expansion of treatment centers are expected to maintain Gaucher disease’s leading role in the market and support continued adoption of targeted enzyme therapies.
- End-User Analysis: Infusion centers dominate the end-user segment with a 45.6% market share, reflecting their critical role in delivering intravenous enzyme replacement therapies. These centers provide specialized care for patients with conditions such as Gaucher disease, enabling safe and efficient administration. Rising demand for outpatient and home-based infusion services is also contributing to their growth. As healthcare systems prioritize accessibility and specialized delivery of biologics, infusion centers will remain pivotal in ERT distribution and administration.
Market Segments
By Product Type
- Imiglucerase
- Agalsidase Beta
- Taliglucerase
- Velaglucerase Alfa
- Others
By Route of Administration
- Parenteral
- Oral
By Therapeutic Condition
- Gaucher Disease
- Pompe Disease
- SCID
- Fabry Disease
- Others
By End-user
- Infusion Centers
- Hospitals
- Others
Regional Analysis
North America led the enzyme replacement therapy market in 2023, accounting for a revenue share of 39.8%. This dominance is attributed to the region’s strong focus on managing rare genetic disorders and the continuous introduction of new therapeutic options. Lysosomal storage disorders (LSDs), which are commonly treated with enzyme replacement therapies, affect approximately 1 in 5,000 newborns, according to the U.S. National Institutes of Health (NIH). The stable incidence of these disorders ensures a consistent demand for lifelong treatments.
Regulatory support further strengthens market growth. In 2023, the U.S. FDA approved two notable therapies: Lamzede (velmanase alfa-tycv) for alpha-mannosidosis in February, and Elfabrio (pegunigalsidase alfa-iwxj) for Fabry disease in May. These approvals have expanded the treatment landscape, offering additional options for patients with specific LSDs.
The Asia Pacific region is projected to register the fastest CAGR during the forecast period. This growth is driven by rising awareness of rare diseases, improved diagnostic infrastructure, and increased healthcare investments. Countries like China are actively cataloging rare diseases, as reflected in national studies and health initiatives. According to the OECD-WHO “Health at a Glance: Asia/Pacific 2024” report, regional healthcare spending continues to rise, supporting greater access to enzyme replacement therapies across the region.
Emerging Trends
- Expansion of Indications and Products: The number of distinct enzyme replacement therapies approved by the U.S. Food and Drug Administration has steadily increased since the first ERT for Gaucher disease in 1991, reaching 20 approved therapies by September 2023. New indications now include alpha-mannosidosis (Lamzede approved February 17, 2023) and hypophosphatasia (Asfotase alfa approved October 2015), broadening treatment to additional rare metabolic disorders.
- Longer-acting and Plant-based Formulations: Next-generation ERTs with extended half-lives have been developed to reduce infusion frequency. For example, pegunigalsidase alfa (Elfabrio) demonstrates an initial circulating half-life of approximately 78.9 hours, permitting dosing intervals of every two to four weeks. Plant-cell–expressed enzymes have thus been employed to enhance stability and patient convenience.
- Efforts to Improve Tissue Penetration and CNS Delivery: Current intravenous ERT formulations achieve significant clearance of substrate in visceral organs but show limited effect in cartilaginous tissues and cannot cross the blood–brain barrier. Studies are under way to combine ERT with other modalities or to modify enzymes for better tissue uptake. For instance, the addition of pharmacological chaperones is being explored to facilitate enzyme folding and distribution.
- Development of Biosimilars and Cost-Reduction Strategies: As original ERT products reach patent expiry, biosimilar versions (e.g., imiglucerase biosimilars for Gaucher disease) are under evaluation to lower treatment costs and increase accessibility. Regulatory pathways have been established to ensure biosimilarity without clinical efficacy loss.
Use Cases
- Gaucher Disease (β-Glucocerebrosidase Deficiency)
- Prevalence: Occurs in approximately 0.9 per 100,000 people worldwide (range 0.7–1.75).
- ERT Impact: Imiglucerase and related formulations reduce spleen and liver enlargement by over 20% within 12 months of treatment initiation.
- Dosage Example: A common regimen is 60 units/kg administered intravenously every two weeks, adjusted based on clinical response and biomarker levels.
- Fabry Disease (α-Galactosidase A Deficiency)
- Patient Numbers: Approximately 11,000 individuals with confirmed Fabry disease are living in the United States.
- ERT Options: Agalsidase beta (Fabrazyme) was first approved in 2003. Pegunigalsidase alfa (Elfabrio) was evaluated in eight international trials involving 142 patients and received FDA approval in May 2023.
- Clinical Outcome: Elfabrio demonstrated significant reduction of globotriaosylceramide (Gb) deposits in kidney tissue after six months, which is expected to slow renal disease progression.
- Pompe Disease (Acid α-Glucosidase Deficiency)
- Incidence: Varies by ethnicity, with overall estimates of about 1 in 40,000 people in the U.S. and up to 1 in 17,000 births in newborn-screened populations.
- ERT Products: Alglucosidase alfa (Myozyme) was approved in 2006 for infantile onset Pompe disease. Subsequent approvals include avalglucosidase alfa (Nexviazyme) in August 2021 and cipaglucosidase alfa in 2023.
- Survival Benefit: In studies of perinatal and infantile-onset Pompe, 97% of treated infants were alive at one year of age compared to 42% in historical controls.
- Mucopolysaccharidosis (MPS) Types I–VII
- Disease Coverage: ERT is available for MPS I (Aldurazyme®), MPS II (Elaprase®), MPS IV A (Vimizim®), MPS VI (Naglazyme®), and MPS VII (Vestronidase alfa under evaluation).
- Clinical Effects: Treatments have been shown to reduce urinary glycosaminoglycan levels by 50–70% and to decrease liver and spleen volume by 30–50% over the first year of therapy.
Conclusion
The enzyme replacement therapy (ERT) market is positioned for strong and sustained growth, supported by increasing prevalence of rare genetic disorders, technological advancements, and expanding treatment approvals. North America maintains market leadership due to advanced healthcare infrastructure, while Asia Pacific emerges as the fastest-growing region.
Continued innovations in formulation, delivery, and biosimilar development are expected to improve access and patient outcomes. With a projected CAGR of 9.1% through 2034, the market is driven by rising demand for lifelong treatments, regulatory support, and growing awareness. ERT remains a vital solution for managing lysosomal storage disorders and other enzyme-deficient conditions globally.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



