Table of Contents
Overview
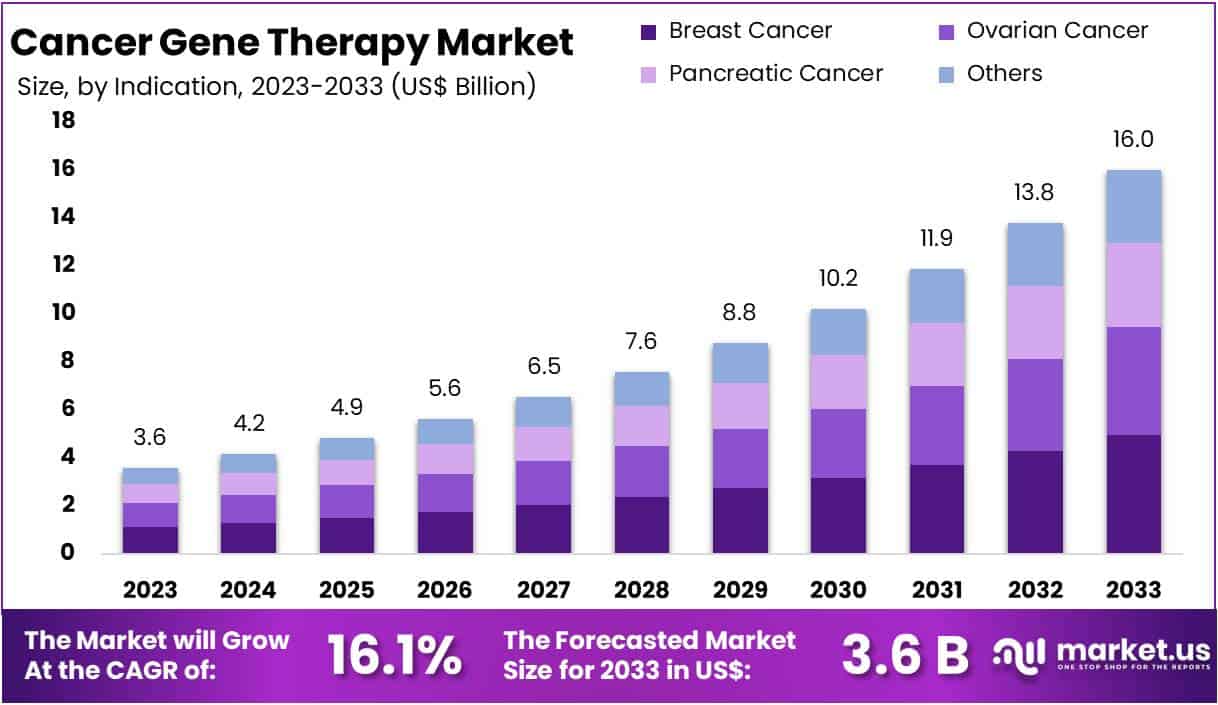
New York, NY – July 24, 2025 – The Global Cancer Gene Therapy Market size is expected to be worth around US$ 16 Billion by 2033, from US$ 3.6 Billion in 2023, growing at a CAGR of 16.1% during the forecast period from 2024 to 2033. North America emerged as the dominant region, securing over 36.5% of the market share with a valuation of US$ 1.3 billion for the year.
Cancer gene therapy, an innovative approach aiming to modify or manipulate genetic material within cancer cells or the surrounding tissue, is gaining momentum as a promising strategy in oncology. This therapy seeks to either correct defective genes or introduce new genes to combat cancer development and progression.
Recent advances in viral and non-viral vector technologies have enabled the precise delivery of therapeutic genes to specific tumor sites. Techniques such as CRISPR-Cas9, oncolytic virotherapy, and RNA interference (RNAi) are now being evaluated in clinical trials, with early results indicating enhanced tumor suppression and improved patient outcomes.
The approach is being explored across various cancer types, including lung, breast, pancreatic, and brain cancers, where traditional treatments such as chemotherapy and radiation have limited efficacy. According to data from the National Cancer Institute (NCI), gene therapy-based interventions are showing reduced toxicity and greater tumor selectivity compared to conventional modalities.
Despite regulatory and technical challenges, continued investment in research and increasing FDA approvals for gene therapy trials underscore its growing clinical potential. As healthcare shifts toward personalized medicine, cancer gene therapy is expected to play a vital role in shaping the future of targeted cancer care.
Key Takeaways
- The Global Cancer Gene Therapy Market is projected to reach approximately US$ 16 billion by 2033, expanding at a compound annual growth rate (CAGR) of 16.1% over the forecast period.
- In 2023, Gene Induced Immunotherapy emerged as the dominant therapy segment, accounting for 38.22% of the total market share, reflecting its growing clinical relevance and therapeutic effectiveness.
- Within the indication segment, Gene Induced Immunotherapy also held a leading position in 2023, representing 31.51% of the overall share, indicating its wide applicability across multiple cancer types.
- Biopharmaceutical companies were identified as the primary end users in 2023, capturing 48.62% of the Cancer Gene Therapy Market, driven by their active involvement in gene therapy research, development, and commercialization.
- North America maintained its position as the leading regional market in 2023, securing a 36.5% share, with a market valuation of approximately US$ 1.3 billion, supported by advanced healthcare infrastructure and favorable regulatory support.
Segmentation Analysis
- Therapy Segment Analysis: In 2023, Gene Induced Immunotherapy dominated the therapy segment with a 38.22% market share, driven by its ability to enhance immune response and support personalized cancer care. Its growing role in long-term remission and ongoing biotech innovations are fostering wider adoption. Oncolytic Virotherapy is also gaining momentum due to its dual action directly destroying cancer cells and activating immune defense. Additionally, Gene Transfer therapy is advancing rapidly with gene editing tools like CRISPR, improving precision and therapeutic outcomes.
- Indication Segment Analysis: Gene Induced Immunotherapy led the indication segment in 2023, securing over 31.51% of the market due to its effectiveness in mobilizing the immune system against cancer. Breast cancer remains the largest application area, supported by increased diagnosis rates and demand for advanced therapies. Ovarian and pancreatic cancers are also key targets, benefiting from gene therapy’s personalized approach and the urgent need for more effective treatments, especially in addressing aggressive and hard-to-treat cancer types.
- End Use Segment Analysis: Biopharmaceutical companies held the largest share of the end use segment in 2023, accounting for 48.62% of the market. Their leadership is supported by robust investments in R&D and the development of cutting-edge gene therapies. Research institutes contribute foundational knowledge and frequently partner with industry leaders to commercialize breakthroughs. Diagnostic centers play a vital role in identifying eligible patients through genetic profiling, reinforcing the personalized nature of gene therapy and optimizing treatment outcomes.
Market Segments
By Therapy
- Gene Induced Immunotherapy
- Oncolytic Virotherapy
- Gene Transfer
By Indication
- Breast Cancer
- Ovarian Cancer
- Pancreatic Cancer
- Others
By End Use
- Biopharmaceutical Companies
- Research Institutes
- Diagnostic Centers
- Others
Regional Analysis
In 2023, North America emerged as the leading region in the global Cancer Gene Therapy Market, accounting for over 36.5% of the total market share, with a valuation of approximately US$ 1.3 billion. This dominance is primarily attributed to the high prevalence of cancer, which drives the need for advanced and targeted treatment options.
The region benefits from a well-established healthcare infrastructure, significant biotechnology investments, and a strong base of biopharmaceutical companies actively engaged in gene therapy research and development. These factors, combined with the presence of world-renowned research institutions and access to advanced technologies, have positioned North America at the forefront of gene therapy innovation.
Furthermore, supportive government initiatives, favorable regulatory policies, and sustained public and private funding have accelerated clinical development and streamlined the approval process for new therapies. The U.S. Food and Drug Administration (FDA) plays a critical role in facilitating faster market entry for gene therapies through clear regulatory pathways.
High levels of patient awareness and participation in clinical trials also contribute to the region’s leadership, enabling more efficient data collection and faster clinical validation. Collectively, these strengths underscore North America’s pivotal role in advancing the Cancer Gene Therapy Market and shaping the future of personalized oncology treatments.
Emerging Trends
- mRNA-based delivery systems: The use of messenger RNA (mRNA) to carry therapeutic genes has expanded rapidly. mRNA platforms enable flexible design of payloads and reduced risk of insertional mutagenesis compared with DNA vectors.
- Nanoparticle carriers for targeted delivery: Lipid- and polymer-based nanoparticles are increasingly employed to protect gene payloads in the bloodstream and direct them to tumor sites, improving uptake and reducing off-target effects.
- Advanced viral vector engineering: Novel viral vectors such as next-generation adenoviruses and lentiviruses are being optimized for higher gene-transfer efficiency and enhanced safety, with modifications that limit replication in noncancerous cells.
- CRISPR/Cas9 gene editing for personalized immunotherapy: CRISPR/Cas9 is being used to precisely edit patient T cells (e.g., knocking out PD-1 or inserting tumor-specific receptors), enabling tailored treatments and the potential to overcome tumor resistance.
Use Cases
- CAR T-cell therapy for blood cancers: Patient T cells are harvested, genetically modified to express a chimeric antigen receptor (CAR), expanded to hundreds of millions of cells, and reinfused. Six CAR T therapies have gained FDA approval since 2017, and the overall process requires about 3–5 weeks per patient.
- T-cell receptor (TCR) therapy for solid tumors: Afamitresgene autoleucel (afami-cel) was approved to treat metastatic synovial sarcoma. In a 44-patient trial, 43 % experienced tumor shrinkage, and the median duration of response was 6 months.
- GD2-targeted CAR T against diffuse midline gliomas: In a small trial of 11 children/young adults, GD2-CAR T infusions directly into the brain led to tumor reduction, with several participants surviving more than 2 years post-treatment.
- CRISPR-engineered T cells in early clinical testing: A first-in-human Phase I trial edited patient T cells using CRISPR/Cas9 to remove endogenous TCR genes and PD-1. Three patients with refractory cancer tolerated the treatment, demonstrating feasibility and safety.
Conclusion
The global Cancer Gene Therapy Market is undergoing rapid transformation, driven by advancements in gene editing technologies, growing clinical success, and rising demand for personalized cancer treatment. With a projected market size of US$ 16 billion by 2033 and a strong CAGR of 16.1%, the sector shows high growth potential.
North America remains the leading region due to robust infrastructure and favorable regulatory support. Innovations such as mRNA delivery, CRISPR-based therapies, and nanoparticle systems are expanding therapeutic possibilities. As research progresses, cancer gene therapy is expected to become a cornerstone of precision oncology, offering safer and more effective treatment options globally.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



