Table of Contents
Overview
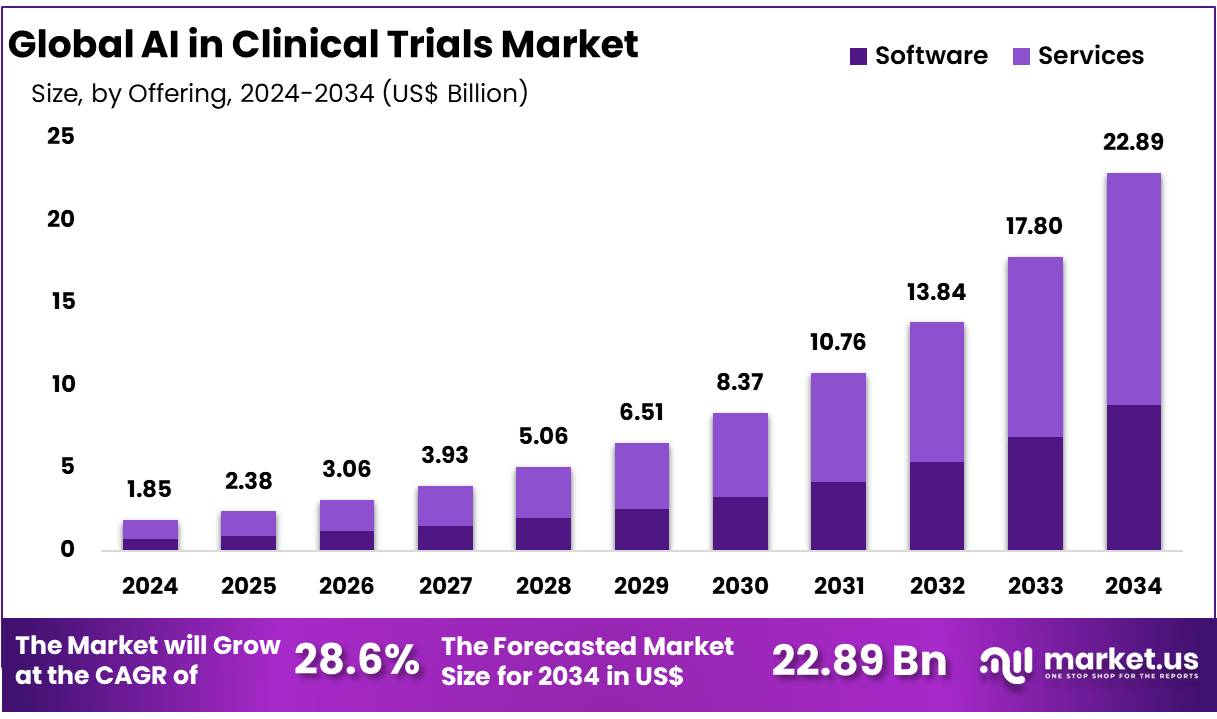
New York, NY – Dec 16, 2025 – Global AI in Clinical Trials Market size is expected to be worth around US$ 22.89 Billion by 2034 from US$ 1.85 Billion in 2024, growing at a CAGR of 28.6% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 31.5% share with a revenue of US$ 582.75 Billion.
The integration of artificial intelligence (AI) into clinical trials is increasingly transforming the way clinical research is designed, conducted, and analyzed. AI-driven technologies are being adopted across multiple stages of clinical trials, including patient recruitment, trial design, data management, and outcome analysis, with the objective of improving efficiency, accuracy, and timelines.
AI-powered algorithms are being widely used to identify eligible patients through advanced analysis of electronic health records, genomic data, and real-world evidence. This approach is helping to reduce recruitment timelines and enhance patient diversity, which remains a critical challenge in traditional clinical trial models. In addition, AI-based predictive analytics is supporting optimized trial design by identifying potential risks and improving protocol feasibility at early stages.
The use of machine learning and natural language processing is also strengthening data monitoring and analysis. Large volumes of structured and unstructured clinical data can be processed in real time, enabling faster detection of safety signals and deviations. As a result, trial quality and regulatory compliance can be improved while operational costs are reduced.
The growing adoption of AI in clinical trials is being supported by increasing investments from pharmaceutical companies, biotechnology firms, and contract research organizations. Regulatory agencies are also actively exploring frameworks to ensure responsible and transparent use of AI-driven tools in clinical research.
Overall, the application of AI in clinical trials is expected to enhance decision-making, accelerate drug development timelines, and support the delivery of innovative therapies to patients more efficiently.
Key Takeaways
- Market Size: The AI in Clinical Trials market was valued at USD 1.85 billion in 2024 and is expected to expand significantly, reaching approximately USD 22.89 billion by 2034.
- By Offering Analysis: The services segment accounted for the largest share of the market, capturing 61.3% of total revenue.
- Technology Analysis: The deep learning segment dominated the market, representing 54.7% of the overall market share in 2024.
- Application Analysis: The oncology application segment emerged as the leading contributor, holding 45.9% of the market share.
- End User Analysis: Pharmaceutical companies constituted the primary end users, accounting for a substantial 65.8% share of the market in 2024.
- Regional Analysis: North America led the global market, with a dominant share of 31.5% in 2024.
Regional Analysis
North America emerged as the leading region in the global AI in clinical trials market, accounting for a significant market share of 31.5% in 2024. This dominance is largely attributed to the strong presence of established pharmaceutical companies such as Abbott Laboratories, Johnson & Johnson, and Pfizer, along with rising research and development expenditures. The region also benefits from a large and well-developed network of clinical trial service providers and increased investments in biosimilars and biologics, which have supported the adoption of AI-driven solutions in clinical research.
In addition, continuous innovation by regional startups has reinforced market growth. For example, bfLEAP, a proprietary artificial intelligence platform developed by U.S.-based startup Bullfrog AI, has been introduced to advance precision medicine by improving data-driven decision-making in clinical trials.
Meanwhile, the Asia-Pacific region is expected to register the fastest growth rate, projected at 31.5% during the forecast period. This accelerated expansion is supported by the growing adoption of AI-based tools, favorable government initiatives promoting AI integration across healthcare, and increasing clinical trial activity. Moreover, the region offers a large and diverse patient population and comparatively lower trial costs, resulting in higher clinical trial enrollment levels than those observed in Europe and North America.
Use cases
- Protocol design optimization
- What AI does: Scans past trial protocols and outcomes to suggest fewer visits, fewer tests, and clearer endpoints.
- Why it matters (numbers): More than 40% of trials were reported to amend the protocol before the first subject visit, causing about 4 months of delay.
- Typical KPI targets: fewer amendments, shorter start-up time, fewer procedures per patient.
- Inclusion/exclusion criteria tuning
- What AI does: Tests criteria rules against real patient data (EHR/RWD) to see how many eligible patients actually exist.
- Why it matters (numbers): ~80% of trials were reported to fail the initial enrollment target and timeline (recruitment is a major cause).
- Typical KPI targets: higher screen-to-enroll rate, lower screen failure rate, faster enrollment curve.
- Feasibility forecasting
- What AI does: Predicts if the trial can recruit enough patients in each country/site based on patient pools and competition.
- Why it matters (numbers): 11% of research sites were reported to enroll zero participants, and 37% under-enroll.
- Typical KPI targets: fewer non-performing sites, higher enrollment per active site.
- Site selection and site ranking
- What AI does: Ranks sites using past enrollment performance + local patient availability signals.
- Why it matters (numbers): A real-world dataset example showed 19% of investigator sites enrolled only one patient, contributing <3% of enrolled patients; meanwhile the top 16% of sites delivered 54% of enrollments.
- Typical KPI targets: higher patients/site, fewer low-output sites, shorter time to full enrollment.
- Patient finding from EHRs
- What AI does: Uses rules + natural language processing to read clinical notes and match patients to eligibility criteria.
- Measured impact (numbers): An AI screener (ACTES) reduced patient screening time by 34% and improved patient enrollment by 11.1%.
- Typical KPI targets: faster prescreening, higher “approached-to-consented” rate, lower coordinator workload.
- Recruitment acceleration and prediction
- What AI does: Predicts enrollment in real time and flags sites that are likely to miss targets.
- Measured impact (numbers): Industry pilot analysis reported AI/ML can boost enrollment by ~10–20% and predict enrollment performance for earlier intervention.
- Typical KPI targets: fewer enrollment stalls, shorter “time to last patient in”.
- Diversity and representation targeting
- What AI does: Maps where eligible patients live (geo + care pathways) and suggests site locations and outreach channels.
- Why it matters (numbers): When many sites under-enroll (e.g., 37%) and some enroll none (11%), broadening effective site coverage becomes critical.
- Typical KPI targets: share of enrolled patients from priority groups, balanced recruitment by region.
- Trial start-up automation
- What AI does: Drafts essential documents, checks completeness, and flags missing data for faster activation.
- Why it matters (numbers): Delays are expensive: updated Tufts analysis indicates mean direct cost per day is about $40,000/day for Phase II–III, with Phase III ~$55,716/day and Phase II ~$23,737/day.
- Typical KPI targets: shorter site activation time, fewer rework cycles, fewer missing documents.
Frequently Asked Questions on AI in Clinical Trials
- How does AI improve patient recruitment in clinical trials?
AI improves patient recruitment by analyzing electronic health records, genetic data, and real-world evidence to identify eligible participants more accurately. This reduces recruitment timelines, minimizes dropouts, and improves patient diversity in trials. - Can AI reduce clinical trial costs?
AI helps reduce clinical trial costs by automating data management, optimizing trial design, and predicting patient outcomes. These efficiencies lower operational expenses, reduce trial failures, and shorten development timelines for new therapies. - How is AI used in clinical trial data management?
AI is applied to clinical trial data management through automated data cleaning, anomaly detection, and real-time monitoring. This improves data quality, ensures regulatory compliance, and allows sponsors to identify risks early in the trial process. - What role does AI play in clinical trial monitoring?
AI supports remote and risk-based monitoring by continuously analyzing trial data for inconsistencies or safety concerns. This approach reduces the need for on-site visits and enables proactive intervention, improving patient safety and trial reliability. - Is AI adoption in clinical trials regulated?
AI use in clinical trials is regulated under existing clinical research and data protection frameworks. Regulatory authorities require transparency, validation, and explainability of AI models to ensure patient safety, ethical compliance, and data integrity. - Which technologies dominate the AI in clinical trials market?
Machine learning, natural language processing, and predictive analytics dominate the market. These technologies are widely used for patient matching, protocol optimization, and clinical data interpretation, supporting more efficient and accurate trial outcomes. - Who are the primary end users in the AI in clinical trials market?
Pharmaceutical companies, biotechnology firms, contract research organizations, and academic research institutions are the primary end users. These stakeholders adopt AI solutions to improve trial productivity, regulatory compliance, and decision-making efficiency. - What is the future outlook for the AI in clinical trials market?
The market outlook remains cautiously optimistic, supported by increasing digital transformation in healthcare and favorable regulatory initiatives. Continued innovation and validation of AI tools are expected to drive sustained adoption across global clinical research ecosystems.
Conclusion
The integration of artificial intelligence into clinical trials is reshaping the clinical research landscape by improving efficiency, accuracy, and decision-making across the trial lifecycle. AI-driven solutions are enabling faster patient recruitment, optimized protocol design, improved data management, and more effective monitoring, resulting in reduced timelines and operational costs.
Strong investments from pharmaceutical companies, CROs, and technology providers, along with supportive regulatory exploration, are accelerating adoption. With North America leading adoption and Asia-Pacific showing rapid growth, the market outlook remains cautiously optimistic. Overall, AI is positioned as a critical enabler of faster, higher-quality, and more patient-centric clinical development.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



