Table of Contents
Overview
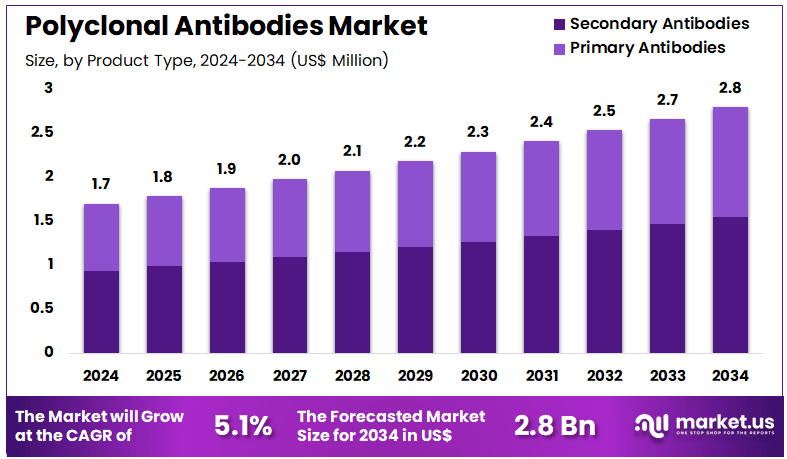
New York, NY – Nov 20, 2025 – Global Polyclonal Antibodies Market size is expected to be worth around US$ 2.8 Billion by 2034 from US$ 1.7 Billion in 2024, growing at a CAGR of 5.1% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 39.8% share with a revenue of US$ 0.7 Billion.
The global polyclonal antibodies market has been experiencing steady expansion as demand for effective research tools and diagnostic solutions continues to rise. Polyclonal antibodies are produced by immunizing host animals with specific antigens, resulting in a heterogeneous mixture of antibody molecules that recognize multiple epitopes on the same target. This broad binding capability has been valued across research applications, including protein detection, signaling pathway analysis, and immunodiagnostics. The versatility of these antibodies has supported their increasing utilization in both academic and commercial laboratories.
Market growth has been influenced by the rising prevalence of chronic and infectious diseases, which has accelerated research activities and diagnostic development. The expansion of proteomics and genomics workflows has also contributed to the increasing adoption of polyclonal antibodies, as these products are widely used for target validation and biomarker identification. The availability of cost-effective production processes and shorter development timelines, compared with monoclonal antibodies, has further strengthened market penetration.
The demand for high-quality antibodies for vaccine development, therapeutic monitoring, and immunoassays has been reinforced by ongoing innovation in biomedical sciences. The growth of biotechnology companies and escalating investments in life-science research have also supported market momentum. As new antigen-generation technologies and purification methods are introduced, the performance and specificity of polyclonal antibodies are expected to advance further. Overall, sustained research funding and continuous scientific progress are anticipated to create favorable opportunities for market expansion in the coming years.
Key Takeaways
- In 2024, the polyclonal antibodies market generated US$ 1.7 billion in revenue, with a CAGR of 5.1%, and is projected to reach US$ 2.8 billion by 2033.
- The product type segment is categorized into primary antibodies and secondary antibodies, with secondary antibodies accounting for 55.2% of the market share in 2023.
- By technology, the market is segmented into rabbits, goats, sheep, mouse, and others, with the diagnostics segment representing a notable 34.5% share.
- In terms of application, the market is divided into biomedical research and diagnostics, where the rabbits sector dominated by capturing 57.7% of total revenue.
- The end-user segment includes pharmaceutical & biotechnology companies, hospitals & diagnostic centers, and academic & research centers, with hospitals & diagnostic centers leading at 53.2% revenue share.
- North America emerged as the leading region, holding 39.8% of the market share in 2024.
Segmentation Analysis
- Product Type Analysis: In 2023, the secondary antibodies category accounted for 55.2% of total revenue, supported by expanding use in immunoassays, western blotting, and flow cytometry. These antibodies improve assay sensitivity and specificity, making them indispensable in research and diagnostic applications. Rising interest in personalized diagnostics and ongoing growth in the biotechnology industry are expected to sustain demand, particularly within customized testing solutions and high-precision research workflows across pharmaceutical and clinical laboratories.
- Technology Analysis: The diagnostics segment represented 34.5% of the market, driven by rising global demand for accurate and sensitive diagnostic technologies. Polyclonal antibodies sourced from rabbits, goats, mice, and sheep play a crucial role in biomarker detection and immunoassays. Growth in point-of-care testing and continuous innovation in diagnostic platforms are expected to enhance adoption. As healthcare systems emphasize early disease detection and streamlined laboratory operations, the use of polyclonal antibodies in diagnostic procedures is projected to expand further.
- Application Analysis: The rabbits segment accounted for 57.7% of revenue due to their capacity to produce high-quality polyclonal antibodies. Rabbits are preferred in biomedical research because their strong immune response supports reliable antibody generation. These antibodies are widely used in oncology, infectious diseases, and autoimmune research. Increasing demand for targeted scientific studies and the global shift toward advanced diagnostic capabilities are expected to maintain strong reliance on rabbits for antibody production.
- End-User Analysis: Hospitals and diagnostic centers captured a 53.2% share, supported by growing dependence on polyclonal antibodies for clinical diagnostic testing. These facilities apply antibodies in assays for cancer, infectious diseases, and cardiovascular disorders, reinforcing the need for rapid and accurate results. As personalized medicine and early detection become more central to healthcare practices, demand for polyclonal antibody-based testing in clinical environments is anticipated to rise, further strengthening this end-user segment.
Regional Analysis
North America Leading the Polyclonal Antibodies Market
North America accounted for the largest share of the polyclonal antibodies market, capturing 39.8% of total revenue. This leadership position was supported by increased research funding, a higher burden of infectious diseases, and expanding therapeutic applications. The National Institutes of Health (NIH) allocated US$ 5.2 billion to antibody-related research in 2023, reflecting an 8% rise in annual funding. Diagnostic utilization increased as Lyme disease cases grew by 15% in 2023, based on data from the Centers for Disease Control and Prevention (CDC).
Therapeutic innovation advanced with 12 new antibody-based therapies receiving FDA approval in 2023, including several products derived from polyclonal sources. Major pharmaceutical companies reported 10–12% expansions in antibody manufacturing capacity according to SEC disclosures. Additionally, the American Cancer Society indicated a 6% increase in immunotherapy trials involving polyclonal antibodies in 2024. These developments collectively strengthened the market position of North America.
Asia Pacific Expected to Record the Fastest CAGR
The Asia Pacific region is projected to achieve the highest growth rate during the forecast period due to supportive government initiatives and rising healthcare demand. Regulatory approvals for antibody-based diagnostics in China increased by 45% in 2023, reflecting intensified infectious disease surveillance. India invested US$ 150 million in immunology research through its Department of Biotechnology, emphasizing therapeutic advancements.
Japan recorded a 20% increase in antibody therapy–related clinical trials in 2024, particularly for autoimmune diseases. Vaccine-focused research also accelerated, with Southeast Asia reporting 25% higher antibody development activity in 2023. Biotechnology companies expanded production capabilities, with some facilities increasing output by 15%. These factors indicate strong, sustained market expansion across the region.
Frequently Asked Questions on Polyclonal Antibodies
- How are polyclonal antibodies produced?
Production involves immunizing an animal with a specific antigen, allowing a natural immune response to generate diverse antibodies. Serum is then collected, purified, and validated for specificity, enabling cost-effective antibody supply for laboratory, diagnostic, or biopharmaceutical use. - What advantages do polyclonal antibodies offer?
Polyclonal antibodies provide strong signal intensity, high sensitivity, and effective detection of low-abundance targets. Their ability to bind multiple epitopes increases robustness in assays, making them suitable for Western blotting, immunohistochemistry, and rapid diagnostic applications across many research settings. - How do polyclonal antibodies differ from monoclonal antibodies?
Polyclonal antibodies bind several antigenic sites, offering broader detection and higher sensitivity, while monoclonal antibodies recognize a single epitope for greater specificity. Polyclonal antibodies are easier and faster to produce, making them more accessible for general laboratory use. - What limitations are associated with polyclonal antibodies?
Variability between production batches, potential cross-reactivity, and limited long-term reproducibility are key constraints. Because they originate from biological sources, maintaining consistent antibody profiles over time can be challenging, especially for applications requiring high experimental precision. - What applications commonly use polyclonal antibodies?
Polyclonal antibodies are widely employed in immunoassays, pathogen detection, biomarker analysis, and therapeutic research. Their ability to detect multiple epitopes supports reliable identification of complex proteins, making them essential tools in diagnostics, vaccine development, and disease monitoring. - Which industries are the primary end users of polyclonal antibodies?
Major end users include pharmaceutical firms, biotechnology companies, academic research institutes, and diagnostic laboratories. These sectors rely on polyclonal antibodies for drug discovery, biomarker validation, assay development, and large-scale diagnostic testing requirements. - What regions are leading the polyclonal antibodies market?
North America leads due to strong R&D investment, followed by Europe with extensive healthcare infrastructure. Growth in Asia-Pacific is increasing rapidly, supported by expanding biopharmaceutical manufacturing, improving research facilities, and rising focus on precision-based diagnostic technologies. - How is technology influencing the market?
Advancements in purification, antigen design, and expression systems improve antibody quality and yield. Enhanced analytical tools support higher validation accuracy, enabling manufacturers to deliver more consistent products that meet complex research and diagnostic requirements globally.
Conclusion
The global polyclonal antibodies market is projected to maintain steady growth as research intensity, diagnostic needs, and biomedical innovation continue to rise. Expansion has been supported by increasing disease prevalence, growing adoption of advanced immunoassays, and sustained investments in life-science research.
Strong demand from hospitals, diagnostic centers, and biotechnology firms is expected to reinforce market development, while improvements in antigen design and purification technologies enhance product performance. Regional momentum remains driven by North America’s established research ecosystem and Asia Pacific’s accelerating biopharmaceutical capacity. Overall, continued scientific progress and healthcare expansion are anticipated to create favorable long-term market opportunities.


