Table of Contents
Overview
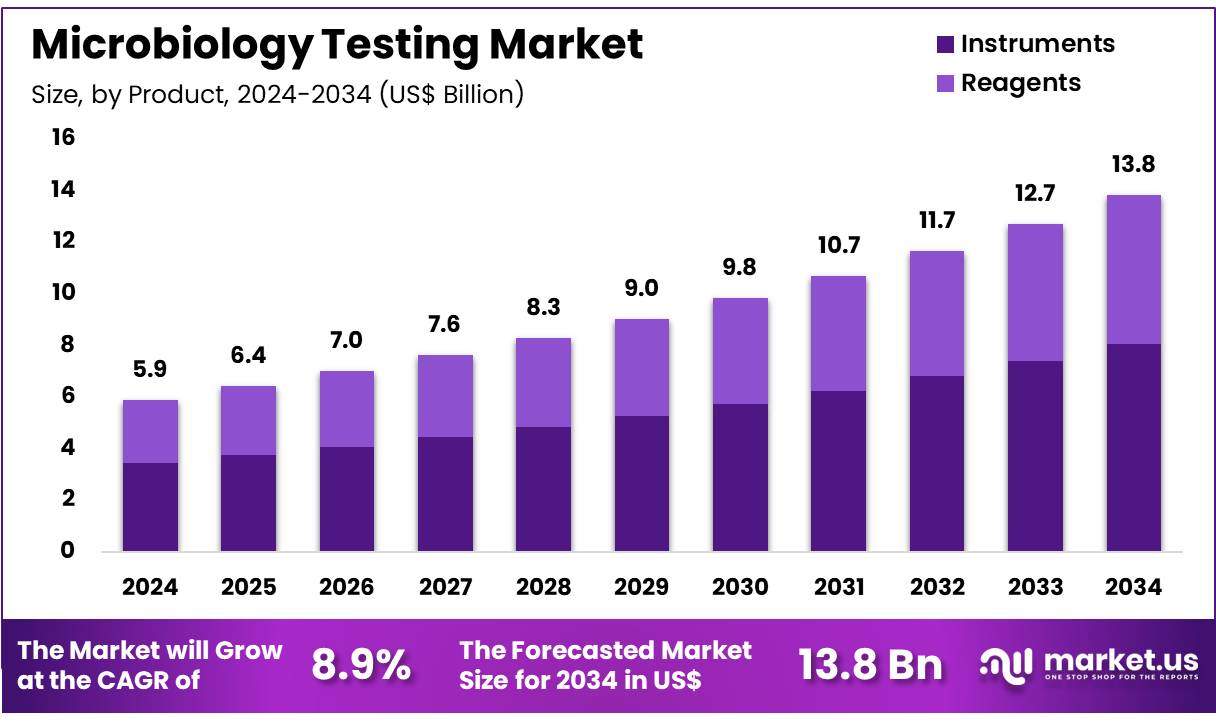
New York, NY – Nov 20, 2025 – Global Microbiology Testing Market size is expected to be worth around US$ 13.8 Billion by 2034 from US$ 5.9 Billion in 2024, growing at a CAGR of 8.9% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 38.8% share with a revenue of US$ 2.3 Billion.
The global microbiology testing landscape has been characterized by steady expansion as demand for rapid, reliable pathogen detection continues to rise. Growth of the market has been attributed to increasing awareness of food safety, heightened regulatory scrutiny, and the continuous emergence of infectious diseases. A stronger focus on preventive healthcare, supported by advanced laboratory automation, has further reinforced adoption across clinical, industrial, and environmental applications.
Significant progress has been observed in culture-based methods, molecular diagnostics, and microbial identification technologies. The integration of polymerase chain reaction (PCR), next-generation sequencing, and mass spectrometry has enabled faster detection times and improved sensitivity. These advancements have supported laboratories in strengthening quality assurance processes and reducing testing turnaround times.
The healthcare sector has maintained a dominant share of the overall revenue due to rising hospital-acquired infections and growing diagnostic volumes. Food and beverage manufacturers have also increased testing output to meet global safety standards. Growth prospects remain strong, as investments in public health infrastructure and biosurveillance systems continue to expand across emerging economies.
Major companies have focused on product innovation, automation, and partnerships to advance testing capabilities. The market is expected to benefit from continued adoption of high-throughput systems and digital laboratory platforms.
Overall, the microbiology testing industry is positioned for sustained advancement, driven by technological improvements, increasing disease surveillance efforts, and a global shift toward preventive, data-driven public health strategies.
Key Takeaways
- In 2024, the Microbiology Testing market generated revenue of US$ 5.9 billion, recorded a CAGR of 8.9%, and is projected to reach US$ 13.8 billion by 2033.
- The product segment includes instruments and reagents, with instruments leading the market in 2024, accounting for 58.3% of total share.
- By test type, the market is classified into bacterial, viral, and fungal tests, with the bacterial segment holding a substantial 54.2% share.
- In terms of application, the market covers respiratory diseases, urinary tract infections, sexually transmitted diseases, periodontal diseases, gastrointestinal diseases, bloodstream infections, and others. The respiratory disease segment dominated with a 41.5% revenue share.
- The end-user segment comprises hospitals, diagnostic centers, academic and research institutes, and others, with hospitals accounting for the largest share at 55.3%.
- North America emerged as the leading regional market, capturing 38.8% of the global share in 2024.
Regional Analysis
North America Leading the Microbiology Testing Market
North America accounted for the largest share of the global microbiology testing market, capturing 38.8% of total revenue. This leadership position can be attributed to continuous technological advancements, rising demand for rapid diagnostic methods, and heightened public awareness of infectious diseases. A significant increase in respiratory illness-related hospital visits during the 2024 flu season, as reported by the Centers for Disease Control and Prevention (CDC), resulted in the highest case levels observed in several years. This rise in infections contributed to an accelerated demand for microbiology testing services.
Market performance was further influenced by industry participants such as QuidelOrtho, which recorded a peak in flu cases affecting their revenue projections. Regulatory activity also supported market expansion. The US Food and Drug Administration (FDA) approved more than 50 biologics and biosimilars in 2023, many of which required microbiology testing for product development, safety assessment, and quality control processes. These approvals emphasized the critical role of microbiology testing in biopharmaceutical research and production.
Additionally, strategic initiatives by the US Department of Health and Human Services (HHS), including the allocation of US$500 million in 2023 for stockpiling essential medical supplies such as diagnostic tools and testing kits, further strengthened regional market growth. These developments collectively reflect the increasing reliance on microbiology testing across healthcare, pharmaceutical, and public health sectors in North America.
What role is played by infectious disease prevalence in shaping demand for microbiology testing?
Infectious disease prevalence plays a central role in determining the demand for microbiology testing because testing volumes rise in direct response to the circulation, emergence, or re-emergence of pathogens. Higher prevalence increases the number of suspected cases, which leads to a proportional rise in diagnostic requests for culture, molecular detection, antimicrobial susceptibility testing, and serological analysis. The growth in testing demand is driven by the need for early detection, accurate pathogen identification, and appropriate therapeutic decision-making.
When infectious diseases become widespread, surveillance activities are expanded, which further contributes to higher utilization of microbiology laboratories. Public health authorities and healthcare institutions typically intensify screening programs during outbreaks, thus increasing routine and emergency diagnostic workloads. The emergence of drug-resistant organisms also strengthens the requirement for detailed microbiological characterization to guide antimicrobial stewardship.
Seasonal infections, such as influenza, and global health threats, such as tuberculosis or new viral pathogens, create cyclical or sustained increases in testing needs. The demand for rapid and high-throughput diagnostic methods is elevated during such periods, as timely results are essential for containment and treatment strategies. Therefore, the prevalence of infectious diseases directly shapes market demand, driving continuous investment in advanced microbiology testing capabilities.
Asia Pacific Expected to Exhibit the Fastest CAGR
The Asia Pacific region is projected to register the highest CAGR over the forecast period. This anticipated growth is driven by rapid expansion of healthcare infrastructure, rising healthcare expenditure, and growing public awareness regarding infection prevention and early disease detection. The Indian Ministry of Health and Family Welfare (MoHFW) reported a 20% increase in government spending on healthcare infrastructure in 2023, with investment directed toward equipping hospitals with advanced diagnostic systems, including microbiology testing technologies.
Similarly, the Chinese National Health Commission (NHC) has prioritized the expansion of diagnostic capabilities, a trend expected to generate substantial demand for microbiology testing solutions. In Japan, the Ministry of Health, Labour, and Welfare (MHLW) allocated US$150 million in 2023 to enhance diagnostic capacity through the procurement of modern testing equipment.
Complementary initiatives in Australia, led by the Department of Health, focused on strengthening healthcare infrastructure and improving access to diagnostic services, particularly in remote communities. These collective investments, combined with an increasing emphasis on early detection and preventive care, are expected to significantly advance the adoption of microbiology testing across the Asia Pacific region.
Use Cases
- Tuberculosis Diagnosis Using Rapid Molecular Assays: Global tuberculosis diagnoses reached 8.2 million cases in 2023, and the rate of bacteriological confirmation increased from 55% in 2018 to 63%. The use of rapid molecular platforms such as Xpert MTB/RIF Ultra and Truenat, which are recommended as initial diagnostic tests, has supported timely case detection. These assays generate results in approximately 80 minutes, and their deployment has strengthened the identification of rifampicin-resistant tuberculosis, particularly among smear-negative and HIV-co-infected individuals.
- Early Detection of Community Infection Trends Through Wastewater Surveillance: The National Wastewater Surveillance System (NWSS) of the CDC oversees pathogen monitoring across more than 1,500 sites in the United States, representing an estimated 151 million individuals or 45% of the national population. Weekly analysis of untreated sewage for pathogens such as SARS-CoV-2, influenza A, and mpox provides early insight into community-level infection dynamics. This approach enables timely public health interventions prior to increases in reported clinical cases.
- Antimicrobial Resistance Monitoring Through GLASS: The Global Antimicrobial Resistance and Use Surveillance System (GLASS) involves 109 participating countries as of 2025 and facilitates the systematic collection of antimicrobial-resistance data from routine clinical specimens. More than 2 million bacterial isolates are aggregated annually. These data support evidence-based national treatment guidelines and inform global antimicrobial stewardship strategies to mitigate escalating resistance trends.
- Standardized Laboratory Data Management with WHONET: The WHONET platform is implemented in over 2,300 microbiology laboratories across more than 130 countries to support standardized management of laboratory data. Global harmonization of antimicrobial susceptibility testing (AST) outputs improves the comparability of resistance trends, strengthens outbreak detection capabilities, and informs public health decisions related to antimicrobial use.
Frequently Asked Questions on Microbiology Testing
- Why is microbiology testing important?
Microbiology testing is essential because it enables early detection of infectious agents, supports effective treatment decisions, and ensures product safety. It also helps prevent outbreaks by providing critical information for surveillance and risk management programs. - What types of tests are included in microbiology testing?
Microbiology testing includes culture-based methods, molecular diagnostics, serology, and antimicrobial susceptibility testing. These methods identify pathogens, detect genetic material, measure immune responses, and determine resistance patterns to guide clinical management. - Which industries rely on microbiology testing?
Healthcare, pharmaceuticals, food and beverage, biotechnology, and environmental testing facilities rely heavily on microbiology testing. These industries use testing to ensure safety, comply with regulations, maintain quality standards, and protect public health. - What factors are driving growth in the microbiology testing market?
Market growth is being driven by rising infectious disease prevalence, increasing antimicrobial resistance, technological advancements, and expanding quality regulations. These factors have encouraged higher testing volumes and continuous investment in advanced diagnostic solutions. - How does automation impact the microbiology testing market?
Automation has improved testing accuracy, reduced manual workloads, and accelerated result turnaround. These improvements have increased laboratory efficiency, strengthened standardization, and contributed to growing adoption of automated microbiology platforms. - What technological trends are influencing the market?
The market is influenced by rapid molecular diagnostics, automation, digital microbiology, artificial intelligence, and point-of-care systems. These technologies enhance accuracy, speed, and data integration, driving broader adoption in clinical and industrial settings. - What is the future outlook for the microbiology testing market?
The future outlook remains positive because ongoing innovation, global health challenges, and rising safety standards are expected to sustain market growth. Continued investment in rapid diagnostics and automated systems will support long-term expansion.
Conclusion
The global microbiology testing market is expected to maintain a strong growth trajectory as infectious disease prevalence, regulatory requirements, and preventive healthcare initiatives continue to expand testing needs. Advancements in molecular diagnostics, automation, and digital laboratory systems have enhanced accuracy and reduced turnaround times, supporting broader clinical and industrial adoption.
Rising healthcare expenditure, particularly in emerging economies, is reinforcing diagnostic capacity and accelerating market penetration. Growing emphasis on biosurveillance, antimicrobial resistance monitoring, and high-throughput platforms further strengthens long-term prospects, positioning the industry for sustained innovation and steady revenue growth across key regions.


