Table of Contents
Overview
New York, NY – Nov 10, 2025 – Global Lateral Flow Assay Market size is expected to be worth around US$ 24.6 Billion by 2034 from US$ 16.8 Billion in 2024, growing at a CAGR of 3.9% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 40.6% share with a revenue of US$ 6.8 Billion.
A lateral flow assay is presented as a rapid diagnostic platform designed to enable point-of-care testing across clinical, pharmaceutical, food safety, and environmental applications. The technology has been characterized by its simplified workflow, as sample introduction, reagent migration, and visual detection occur within a single test strip. The formation of the assay generally involves a sample pad, conjugate pad, nitrocellulose membrane, and absorbent pad aligned in a linear structure to facilitate capillary action.
The sample pad is prepared to condition the specimen and ensure controlled flow. The conjugate pad contains labeled antibodies or antigens that bind to the target analyte. The nitrocellulose membrane incorporates test and control lines, where antigen-antibody interactions generate a visible signal. The absorbent pad maintains consistent flow by drawing the liquid across the membrane. This configuration has supported broad adoption because it allows results to be obtained within 10 to 20 minutes without the need for laboratory infrastructure.
Market demand for lateral flow assays has been driven by the increasing emphasis on decentralized diagnostics, rising prevalence of infectious diseases, and expanding use in chronic condition monitoring. Growth has also been supported by ongoing technological improvements, including enhanced sensitivity, multiplexing capabilities, and integration with digital readers for data capture.
The versatility of the format has encouraged its use in both professional and home-based testing, and its scalability has enabled rapid manufacturing expansion during global health emergencies. Continued investment in assay development, material innovation, and regulatory approvals is projected to strengthen the market outlook over the forecast period.
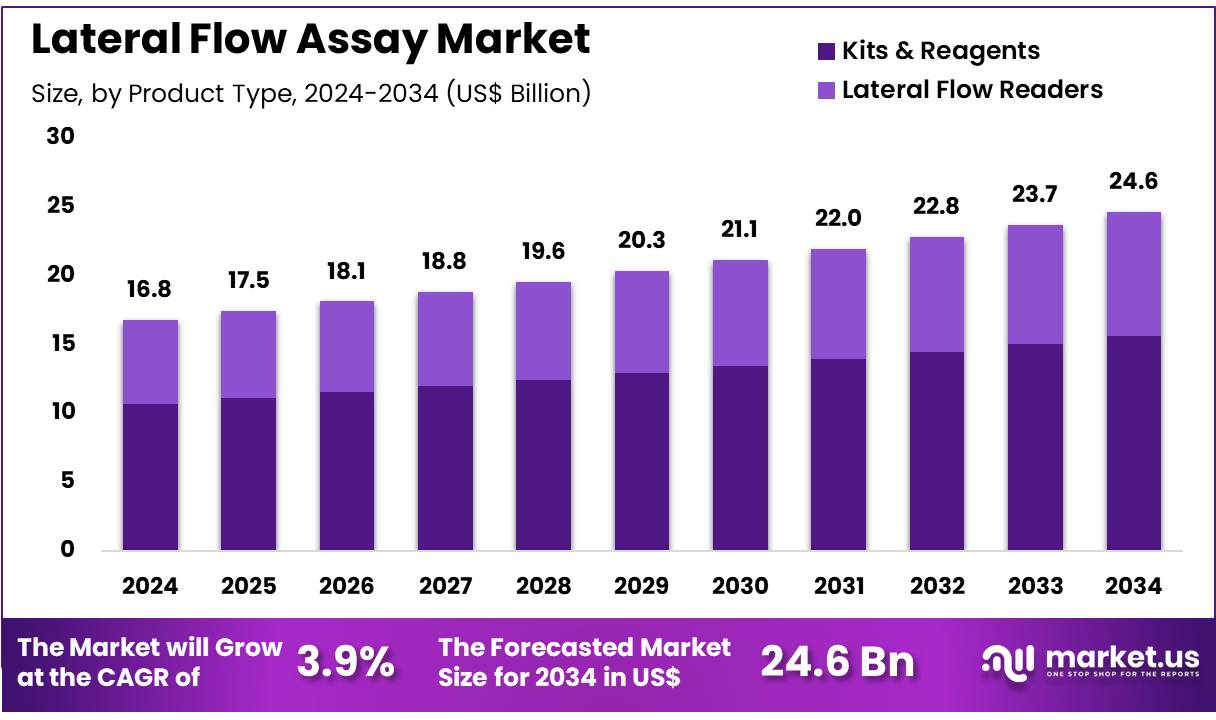
Key Takeaways
- In 2024, the lateral flow assay market generated revenue of US$ 16.8 billion, recorded a CAGR of 3.9%, and is projected to reach US$ 24.6 billion by 2033.
- The product type segment comprises kits and reagents and lateral flow readers, with kits and reagents accounting for 63.5% of the market share in 2023.
- Based on technology, the market is categorized into sandwich assays, competitive assays, and multiplex detection assays, with sandwich assays representing 45.7% of the share.
- In terms of application, the market includes clinical testing, veterinary diagnostics, food safety and environment testing, and drug development and quality testing, with clinical testing holding the largest share at 50.4%.
- The test type segment is divided into lateral flow immunoassay and nucleic acid lateral flow assay, where lateral flow immunoassay contributed 67.3% of the revenue.
- By end user, the market is segmented into hospitals and clinics, diagnostic laboratories, home care, and others, with hospitals and clinics accounting for 48.6% of the share.
- North America dominated the lateral flow assay market in 2023 with a 40.6% share.
Regional Analysis
North America is leading the Lateral Flow Assay Market
North America accounted for the largest revenue share of 40.6%, supported by rising demand for rapid and accessible diagnostic solutions. The availability of at-home COVID-19 rapid tests in the U.S. increased from 24 million units in August 2021 to nearly 300 million units by December 2021, indicating a strong shift toward decentralized testing.
The growing need for rapid screening of infectious diseases such as influenza, RSV, and sexually transmitted infections continued to promote widespread utilization. Improvements in assay sensitivity and specificity enhanced diagnostic reliability, while the expansion of point-of-care testing in pharmacies and healthcare facilities contributed to market growth.
Public health initiatives and government investments in pandemic response further supported adoption. Increased consumer awareness of self-testing and the expansion of online distribution channels improved accessibility. Strategic collaborations between biotechnology and diagnostic companies accelerated next-generation assay development, reinforcing the region’s leadership position.
Asia Pacific is expected to experience the highest CAGR during the forecast period
Asia Pacific is projected to register the fastest growth rate due to expanding healthcare access and an increasing disease burden. Investments in infectious disease surveillance across China, India, and Southeast Asia are expected to support wider use of rapid testing technologies. Government efforts focused on early detection and pandemic preparedness are anticipated to encourage market expansion.
Partnerships between global diagnostic companies and regional manufacturers are likely to enhance product availability and reduce costs. Growing consumer adoption of home-based testing for pregnancy, diabetes, and infectious diseases is expected to drive demand.
Technological advancements such as AI-enabled rapid test readers are projected to improve accuracy and usability. Rising need for affordable diagnostics in rural and underserved areas, combined with the expansion of digital health and telemedicine platforms, is expected to strengthen market growth across the region.
Emerging Trends in Lateral Flow Assays
- Integration of Nanomaterials: The incorporation of advanced nanomaterials, including gold nanoparticles, quantum dots, and upconverting nanoparticles, has strengthened the sensitivity and specificity of LFA platforms. UCNP-based systems have enabled hepatitis B surface antigen detection at 0.1 IU/mL, improving performance compared with traditional 3.2 IU/mL limits.
- Expansion of Multiplexed Detection: Progress in assay design has facilitated multiplexed formats capable of detecting several analytes within a single test. This development has supported disease differentiation, improved clinical efficiency, and enhanced biomarker monitoring across complex diagnostic scenarios.
- Digital and Smartphone Compatibility: The linkage of LFAs with smartphones and digital readers has enabled quantitative interpretation, secure data storage, and remote monitoring. This integration has improved usability in point-of-care environments and strengthened support for telemedicine-based diagnostic services.
- Broader Use in Environmental and Food Safety Testing: Lateral flow technology is increasingly being adopted for environmental surveillance and food safety assessments. Its rapid turnaround time and portability have supported on-site detection of contaminants, toxins, and microbial pathogens across diverse operational settings.
Key Use Cases
- Infectious Disease Diagnosis: Lateral flow assays are widely utilized for rapid detection of infectious agents such as SARS-CoV-2, HIV, and influenza. Their quick results have supported early intervention, timely clinical decisions, and effective containment strategies.
- Pregnancy and Fertility Testing: Home pregnancy and fertility tests rely on LFA technology to identify hormones such as hCG and ovulation-related markers. Their ease of use and quick results have established LFAs as standard tools in home-based reproductive testing.
- Cardiac Marker Assessment: LFAs are used to detect cardiac biomarkers, including troponin, enabling rapid identification of myocardial infarction. Their immediate results support urgent clinical decisions and contribute to improved patient outcomes in emergency settings.
- Drug Screening Applications: Drug testing programs employ LFAs to identify substances of abuse in workplaces, rehabilitation centers, and law enforcement settings. These assays provide fast, non-invasive, and reliable screening outcomes across varied operational environments.
- Veterinary Diagnostic Testing: Veterinary applications utilize LFAs to diagnose infectious diseases in animals, supporting timely treatment decisions and reducing disease transmission. Their rapid performance and ease of use have made them valuable tools in clinical and field-based veterinary services.
Frequently Asked Questions on Lateral Flow Assay
- What is a lateral flow assay?
A lateral flow assay is a rapid diagnostic method used to detect targeted analytes in samples through immunochromatography. The test provides qualitative or semi-quantitative results within minutes, and its adoption has been driven by increasing demand for decentralized diagnostic solutions. - How does a lateral flow assay work?
A lateral flow assay functions through capillary action, where a liquid sample migrates across a strip containing antibodies or reagents. The interaction between the analyte and labeled antibodies forms visible lines, enabling fast detection without complex equipment or technical expertise. - What are the major applications of lateral flow assays?
Applications of lateral flow assays include infectious disease diagnostics, pregnancy testing, veterinary testing, and environmental monitoring. Their use has expanded due to rising demand for rapid testing solutions in remote locations, point-of-care settings, and emergency response programs globally. - What advantages do lateral flow assays provide?
Advantages of lateral flow assays include rapid turnaround time, low operational cost, minimal training needs, and high portability. These attributes have contributed to widespread utilization in low-resource settings, accelerating early diagnosis and increasing accessibility across diverse clinical and non-clinical environments. - What limitations are associated with lateral flow assays?
Limitations of lateral flow assays include lower sensitivity compared with laboratory-based molecular techniques and variability in accuracy due to sample quality. Despite these constraints, continuous technological improvements have enhanced reliability and expanded acceptance across global diagnostic workflows. - Which regions dominate the lateral flow assay market?
North America and Europe currently dominate the lateral flow assay market due to strong healthcare infrastructure, high diagnostic awareness, and significant research investments. Asia-Pacific is gaining momentum as rising healthcare spending and population growth increase diagnostic accessibility across developing economies. - What trends are shaping the future of the lateral flow assay market?
The market is being shaped by trends such as digital connectivity, smartphone-based readers, enhanced sensitivity systems, and multiplexing capabilities. These developments are improving clinical value, supporting decentralized care models, and creating opportunities for manufacturers to expand product portfolios. - Who are the major end users in the lateral flow assay market?
Major end users include hospitals, diagnostic laboratories, home-care settings, and research institutions. Demand from pharmaceutical and biotechnology companies has increased due to the growing use of lateral flow formats in drug development, quality control, and biomarker validation studies.
Conclusion
The lateral flow assay market is positioned for sustained expansion as demand for rapid, decentralized diagnostics continues to rise across clinical, veterinary, environmental, and consumer applications. Growth has been supported by technological innovations that improved sensitivity, enabled multiplexing, and strengthened digital integration.
Regional trends indicate strong adoption in North America and accelerating growth in Asia Pacific due to expanding healthcare access and rising disease surveillance initiatives. Widespread use in infectious disease detection, pregnancy testing, cardiac assessment, and drug screening reinforces its clinical relevance. Continued investment in assay development and material advancement is expected to enhance performance and broaden future market opportunities.


