Table of Contents
Overview
New York, NY – Nov 05, 2025 – Global Anti Infective Endotracheal Tube Market size is expected to be worth around US$ 1.5 billion by 2034 from US$ 0.9 billion in 2024, growing at a CAGR of 5.1% during the forecast period 2025 to 2034. In 2023, North America led the market, achieving over 39.6% share with a revenue of US$ 0.4 Billion.
The Anti-Infective Endotracheal Tube market is gaining significant attention due to the rising incidence of hospital-acquired infections and ventilator-associated pneumonia in intensive care settings. The integration of antimicrobial coatings and advanced design technologies has been instrumental in improving airway management safety and reducing infection risk during mechanical ventilation. A growing preference for infection-control solutions in critical care units has been observed across both developed and emerging healthcare systems.
The growth of the market can be attributed to the increasing burden of respiratory diseases, including chronic obstructive pulmonary disease, acute respiratory distress syndrome, and COVID-19 complications. Government initiatives supporting patient safety protocols and infection prevention measures have further contributed to the increasing adoption of anti-infective tubes in hospitals and surgical centers.
Anti-infective endotracheal tubes are manufactured using specialized polymers and coatings designed to inhibit bacterial growth and biofilm formation. The use of silver ion technology, antibiotic-impregnated surfaces, and other protective compounds has been recognized as an essential advancement in modern intensive care practice. The product is positioned to support improved clinical outcomes, minimize treatment-associated complications, and reduce healthcare system burden associated with extended hospital stays.
Continuous innovation in medical device engineering and strategic collaborations between healthcare manufacturers and clinical research institutions are anticipated to strengthen product availability and further enhance performance capabilities in the coming years. Growing demand for infection-resistant medical devices is expected to sustain steady market expansion.
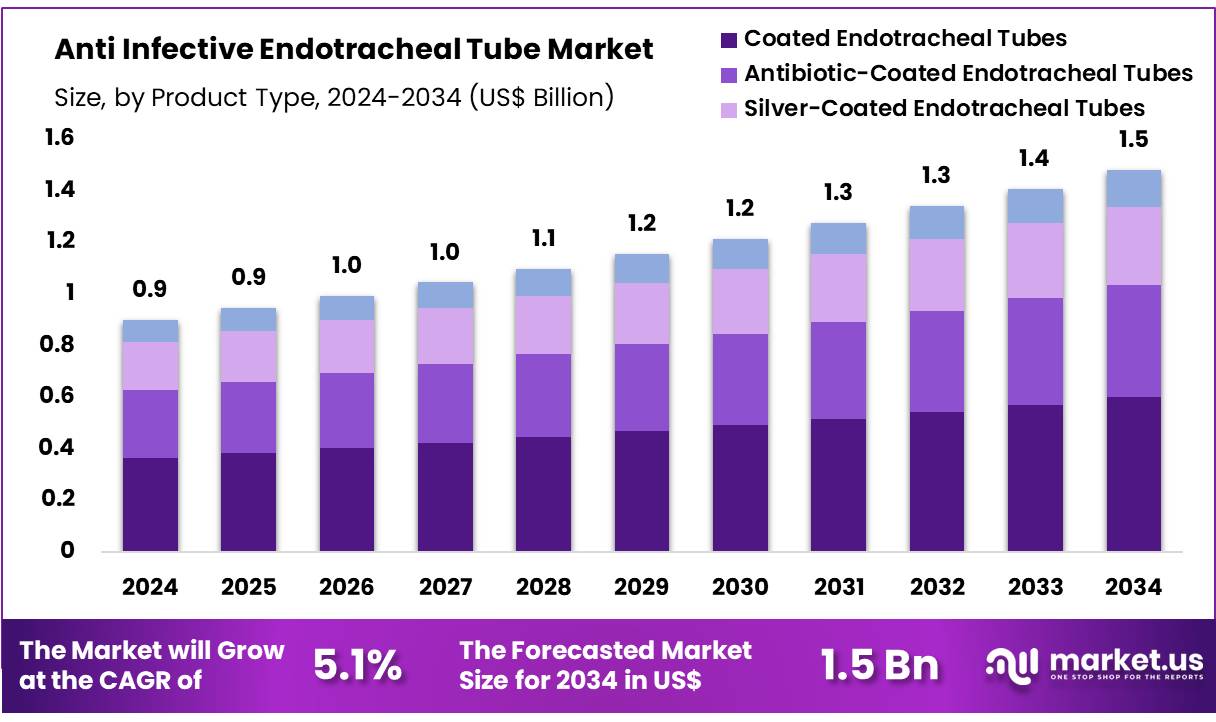
Key Takeaways
- The Anti-Infective Endotracheal Tube market generated revenue of US$ 9 billion in 2024, supported by a compound annual growth rate of 5.1%. The market is projected to reach US$ 1.5 billion by 2033.
- The market has been categorized by product type into coated endotracheal tubes, antibiotic-coated endotracheal tubes, silver-coated endotracheal tubes, and uncoated endotracheal tubes. Coated endotracheal tubes led the segment in 2023 with a market share of 40.5%.
- Based on material, the market includes polyvinyl chloride, polyurethane, silicone, and other materials. Polyvinyl chloride accounted for the largest portion, representing 45.8% of the market.
- With regard to end users, the market is segmented into hospitals, ambulatory surgical centers, and others. Hospitals dominated the market, capturing 53.2% of the total revenue share.
- Regionally, North America held the leading position with a market share of 39.6% in 2023.
Regional Analysis
North America remains the leading region in the Anti-Infective Endotracheal Tube market, accounting for a revenue share of 39.6%. The dominance of the region can be attributed to technological advancements, a rising incidence of respiratory infections, and an increased focus on patient safety in critical care environments.
Anti-infective endotracheal tubes, engineered to minimize infection risks during intubation, have become vital in managing patients requiring mechanical ventilation. The market expansion has been supported by a growing number of surgeries, higher ICU admissions, and intensified efforts to prevent ventilator-associated pneumonia and similar complications.
In May 2021, Medtronic introduced its SonarMed airway monitoring system in the United States, a technology employing acoustic signals to identify tube blockages and confirm placement in real time, thereby enabling timely clinical intervention, particularly in pediatric care.
Asia Pacific is projected to witness the highest growth rate during the forecast period. The anticipated expansion is driven by increased investment in healthcare infrastructure, rising awareness regarding infection prevention, and the growing prevalence of respiratory illnesses. Demand is expected to rise in major countries such as China, India, and Japan, supported by advancements in healthcare systems and a growing volume of patients requiring mechanical ventilation.
Increasing surgical procedures, a surge in chronic respiratory conditions, and expanding intensive care capacities are expected to underpin market demand. Government initiatives focused on improving care quality and safety, combined with the wider adoption of infection-control medical technologies, are likely to strengthen the market outlook. Continuous emphasis on reducing hospital-acquired infections and enhancing patient outcomes is expected to drive considerable growth in the region over the coming years.
Emerging Trends
- Advancement in Antimicrobial Coating Technologies: The progression of antimicrobial coatings for endotracheal tubes has been accelerated to limit bacterial colonization on tube surfaces. Both active biocidal-release coatings and passive anti-adhesion coatings are being adopted. Silver and zinc-oxide nanoparticle coatings have shown biofilm inhibition in preclinical work. These coatings are integrated during manufacturing without altering structural integrity, supporting smoother regulatory review processes.
- Adoption of Subglottic Secretion Drainage Designs: Integrated subglottic secretion drainage lumens are increasingly utilized to remove pooled secretions above the cuff, lowering aspiration risk. Several designs have obtained U.S. FDA clearance through 510(k) pathways due to similarity with predicate devices and recognized VAP-reduction potential. Clinical recommendations now advise using these tubes for patients anticipated to require mechanical ventilation beyond 48 hours.
- Rising Focus on VAP-Prevention Technologies: Heightened awareness of ventilator-associated pneumonia as a significant healthcare-associated infection has guided hospitals toward infection-preventive endotracheal tube designs. CDC data indicated that VAP represented a major share of pneumonia in ventilated patients. As a result, ICUs have aligned procurement strategies with prevention protocols, favoring antimicrobial surfaces and subglottic drainage features.
- Material Innovations to Reduce Microaspiration: New cuff materials such as low-permeability polyurethane have been introduced to reduce microaspiration around the cuff area. While PVC cuffs remain widespread, polyurethane cuffs demonstrated up to 50 percent leakage reduction in bench testing. This reduction in leakage can limit bacterial entry into the lower airways, with clinical validation studies ongoing for long-term patient outcomes.
Use Cases
- ICU Patients on Mechanical Ventilation: Patients requiring prolonged mechanical ventilation remain at elevated risk for VAP. In 2015, hundreds of thousands of patients underwent ventilation, and a notable proportion developed VAP. Subglottic suction tubes demonstrated approximately 40 percent VAP reduction compared to conventional tubes, while antimicrobial-coated devices showed over 90 percent reduction in bacterial adherence in laboratory settings.
- Extended Surgical and Trauma Ventilation Cases: For procedures expected to necessitate postoperative ventilation exceeding 48–72 hours, anti-infective tubes are frequently selected. CDC guidance supports subglottic drainage use in these scenarios. A multicenter analysis reported that in high-risk trauma cases, VAP incidence decreased from 20 percent to 12 percent when subglottic drainage tubes were used during the initial week of ventilation.
- Emergency and Prehospital Intubations: Critically ill patients undergoing urgent intubation, including those with shock, traumatic brain injury, or ARDS, benefit from antimicrobial-coated tubes due to early bacterial colonization risk. With an estimated hundreds of thousands of emergency intubations occurring annually in the United States, coated tubes can limit bacterial attachment during the first 72 hours of ventilation, a recognized high-risk period for VAP onset.
- High-Risk and Immunocompromised Populations: During the COVID-19 pandemic, ventilated SARS-CoV-2 patients experienced higher VAP rates, prompting broader deployment of coated and subglottic drainage tubes. Enhanced oral care practices accompanied this shift. Immunocompromised patients similarly benefited, with controlled studies indicating over 80 percent reduction in biofilm formation on coated devices, offering improved protection in vulnerable groups.
- Pediatric Intensive Care Applications: Pediatric guidance now supports subglottic drainage for cases involving ventilation beyond 72 hours. Evidence from multiple pediatric ICUs reported VAP reductions from approximately 6 percent to 3 percent when antimicrobial-coated tubes were employed. Children’s hospitals have expanded stocking to cover infant through adolescent sizes, reflecting broader recognition of benefit among younger patients.
Frequently Asked Questions on Anti Infective Endotracheal Tube
- How do anti-infective endotracheal tubes work?
The tubes work by releasing antimicrobial substances or using antimicrobial surfaces that prevent biofilm formation. This mechanism reduces bacterial colonization along the airway and helps improve patient outcomes during prolonged intubation or critical care treatment. - What materials are used in anti-infective endotracheal tubes?
These tubes are commonly made from medical-grade polymers combined with antimicrobial agents such as silver ion coatings or antibiotic compounds. The materials are selected for safety, durability, and effective infection-control performance during extended clinical use. - Why are anti-infective endotracheal tubes important in ICU settings?
In ICU environments, patients receiving ventilation are at high risk of hospital-acquired infections. Anti-infective tubes help mitigate infection risks, reduce treatment complications, shorten ICU stays, and improve overall survival outcomes among critically ill patients requiring long-term ventilation. - Are anti-infective endotracheal tubes safe for patients?
These tubes undergo rigorous clinical evaluation to ensure safety and biocompatibility. The antimicrobial coatings are designed to deliver infection prevention without causing tissue toxicity or adverse reactions, supporting safe airway management in intensive care and surgical settings. - What factors are driving the anti-infective endotracheal tube market?
Market growth can be attributed to rising incidence of ventilator-associated infections, increased ICU admissions, enhanced awareness of infection prevention, and technological advancements in antimicrobial coatings. Growing global spending on critical care infrastructure supports continued product adoption. - Which regions dominate the anti-infective endotracheal tube market?
North America and Europe currently lead the market due to strong healthcare systems, high infection-control standards, and advanced ICU facilities. Asia-Pacific is expected to grow rapidly owing to rising healthcare investment and expanding critical care capacities. - Who are the key end-users in this market?
Primary end-users include hospitals, surgical centers, and intensive care units. These facilities prioritize infection-control technologies to manage high-risk patients requiring mechanical ventilation, emergency airway management, or long-term respiratory support in critical care environments. - What future trends are expected in the market?
Future growth will be supported by new antimicrobial technologies, increased adoption of silver-coated and antibiotic-coated devices, and rising emphasis on ICU infection control. Expanded healthcare access and improved hospital infection-prevention frameworks will reinforce long-term market demand.
Conclusion
The Anti-Infective Endotracheal Tube market is positioned for steady expansion as infection prevention in critical care remains a global priority. The rising burden of respiratory diseases, increased ICU admissions, and heightened focus on reducing ventilator-associated pneumonia have accelerated product adoption across advanced and developing healthcare systems.
Technological innovations, including antimicrobial coatings, subglottic drainage features, and enhanced cuff materials, continue to strengthen device performance and clinical outcomes. Supportive government policies, hospital-driven infection-control protocols, and ongoing advancements in medical technology are expected to reinforce market growth, while the shift toward patient-safety-focused solutions is anticipated to sustain long-term demand.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



