Table of Contents
Introduction
The Ursodeoxycholic Acid Drug Product Market Size is projected to reach USD 1,133.8 million by 2033, growing at a CAGR of 11.3% from 2024 to 2033. In 2023, the market was valued at USD 388.7 million. This growth is driven by increasing liver disease prevalence and advancements in UDCA applications, particularly in treating primary biliary cholangitis (PBC) and gallstone diseases.
The rising incidence of liver conditions has significantly boosted the demand for UDCA-based treatments. According to the American Liver Foundation, PBC predominantly affects women aged 35 to 60, with about 65 out of every 100,000 women in the U.S. diagnosed with the condition. Recent advancements, such as the FDA’s approval of Gilead Sciences’ Livdelzi for PBC treatment in combination with UDCA, highlight the drug’s expanding therapeutic scope. Clinical trials show that 62% of patients experienced biochemical improvement within a year of combined therapy.
Regulatory frameworks have also facilitated market growth. The European Medicines Agency (EMA) has provided specific bioequivalence guidelines for UDCA formulations, enabling the entry of generic versions. These clear pathways encourage pharmaceutical companies to develop and expand their UDCA product lines. For example, Latvia-based Grindeks recently increased its UDCA production to target 15% of the global market.
Overall, the UDCA market is driven by growing disease prevalence, evolving therapeutic applications, and regulatory support. Increased production capacities and new drug approvals further strengthen its position in the global pharmaceutical landscape. These trends indicate a strong future for UDCA-based treatments.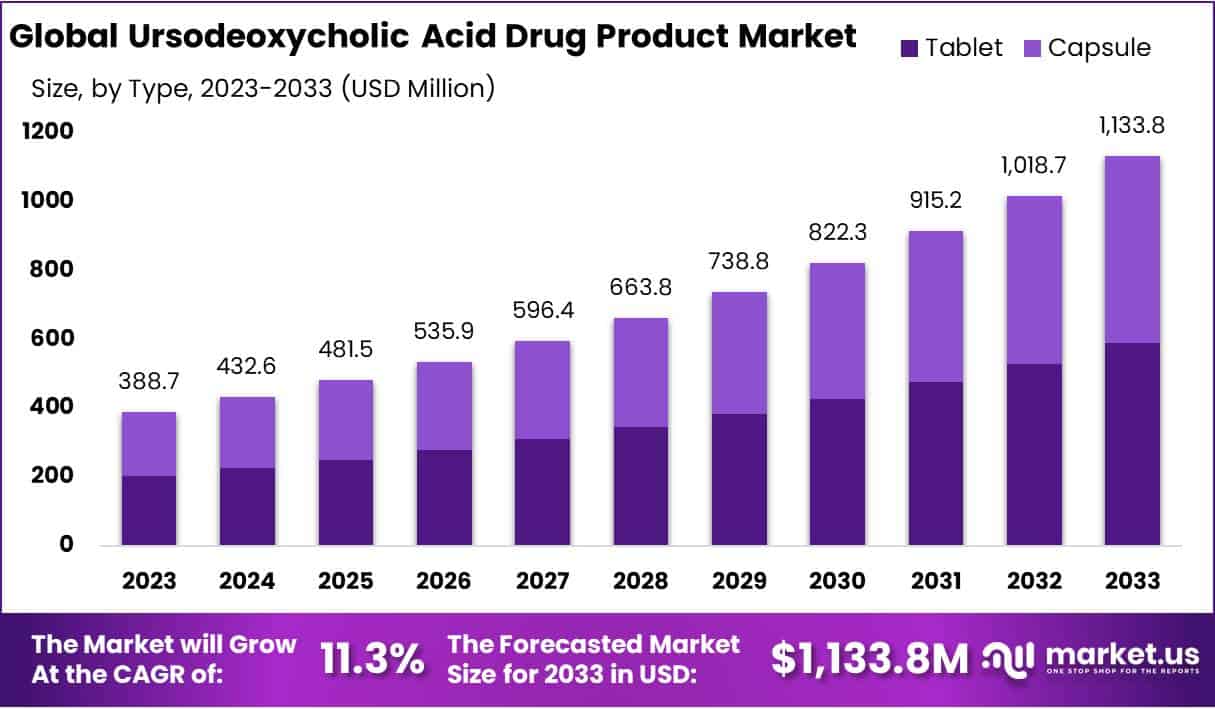
Key Takeaways
- The UDCA market is projected to reach USD 1,133.8 million by 2033, growing at a CAGR of 11.3% from 2024 to 2033.
- Tablets held over 52% market share in 2023 due to convenience, affordability, and precise dosage, making them the preferred choice among patients.
- The primary biliary cholangitis (PBC) segment captured over 59% market share in 2023, driven by rising prevalence and awareness of UDCA’s efficacy.
- Increasing liver disease prevalence, such as NAFLD affecting 80-100 million Americans, significantly drives the demand for UDCA treatments globally.
- Adverse effects and limited efficacy in advanced liver diseases pose challenges to UDCA market growth and adoption rates.
- Intensified research could improve UDCA formulations and explore its use in treating other conditions, expanding the market further.
- The growing popularity of generic UDCA, offering up to 85% cost savings, enhances affordability and access for more patients worldwide.
- North America led the UDCA market in 2023, holding over 36.6% share with a value of USD 142.2 million, driven by advanced healthcare systems.
- Active R&D initiatives and collaborations in North America suggest continued growth, though regulatory shifts might influence market dynamics.
- Innovation, adherence to regulations, and advancing therapeutic options are critical for sustained growth and success in the UDCA market.
Emerging Trends
- Combination Therapies: The U.S. Food and Drug Administration (FDA) approved drugs like Livdelzi (seladelpar) and Iqirvo (elafibranor) for PBC treatment. These drugs are prescribed alongside UDCA for adults who show insufficient response to UDCA alone. They also serve as standalone options for patients who cannot tolerate UDCA. This highlights a growing focus on personalized therapies to improve outcomes in PBC patients.
- Bioequivalence Guidelines: The European Medicines Agency (EMA) introduced specific bioequivalence guidance for UDCA formulations such as capsules, tablets, and suspensions. These guidelines ensure consistent quality and therapeutic benefits across different brands and forms. This initiative supports patient safety and promotes trust in UDCA-based treatments, paving the way for high-quality alternatives in the market.
- Orphan Drug Designation: UDCA received an orphan drug designation from the EMA for treating Niemann-Pick disease, a rare lysosomal storage disorder. This recognition incentivizes further research into using UDCA for rare diseases. It also encourages pharmaceutical companies to explore innovative applications for UDCA, potentially expanding its use beyond liver-related conditions.
Use Cases
- Primary Biliary Cholangitis (PBC): UDCA is the first-choice treatment for managing Primary Biliary Cholangitis (PBC), a long-term liver disease. It works by improving liver enzyme levels and slowing the disease’s progression. Nearly 60% of PBC patients show a positive response to UDCA therapy. Regular use can help in reducing liver inflammation and damage over time. However, for patients unresponsive to UDCA, additional treatments may be necessary. Its effectiveness and tolerability make UDCA a standard option for this condition.
- Gallstone Dissolution: UDCA helps dissolve small cholesterol gallstones in people unable to undergo surgery. It lowers cholesterol saturation in bile, which gradually reduces gallstones. The treatment is non-invasive and effective for specific types of gallstones. However, once treatment stops, gallstones may return. This use is ideal for patients with no surgical options or those preferring a medical approach. Regular monitoring is crucial to track progress during therapy.
- Intrahepatic Cholestasis of Pregnancy (ICP): UDCA is widely used for managing Intrahepatic Cholestasis of Pregnancy (ICP). This liver condition in pregnancy causes itching and can lead to complications. UDCA reduces itching in pregnant women and might lower the chance of preterm births. Its effects on fetal distress or other outcomes are likely minimal. It is considered safe for use during pregnancy under medical supervision. This makes UDCA a preferred choice for improving maternal comfort in ICP.
- Cystic Fibrosis-related Liver Disease: UDCA is sometimes used in cystic fibrosis patients to improve bile flow and lower liver enzyme levels. It may help in mild cases but lacks strong evidence for routine use. Long-term benefits, like reducing death rates or preventing liver transplants, remain unclear. Due to limited data, its role in cystic fibrosis treatment is still debated. Doctors typically recommend it only in select cases after evaluating risks and benefits.
Conclusion
The Ursodeoxycholic Acid (UDCA) drug product market is poised for substantial growth, driven by increasing liver disease prevalence and innovative therapeutic applications. Advancements in combination therapies and bioequivalence guidelines have enhanced treatment options and market accessibility. North America leads the market due to advanced healthcare systems and ongoing research, while regulatory support in Europe fosters growth and innovation. Challenges such as adverse effects and limited efficacy in advanced cases persist, but intensified research and the rising popularity of generic options enhance affordability and accessibility. With its expanding therapeutic potential and robust demand, UDCA remains a vital component in addressing liver-related and other medical conditions, indicating a promising future for this market.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



