Table of Contents
Ischemic Heart Disease Treatment Market Overview
Treatment for ischemic heart disease (IHD) includes lifestyle changes, medications, and surgical options. Important lifestyle modifications involve adopting a heart-healthy diet, increasing physical activity, quitting smoking, and achieving a healthy weight.
Medications such as antiplatelet agents, statins, beta-blockers, ACE inhibitors, nitrates, and calcium channel blockers help manage symptoms and risk factors.
To enhance blood flow, surgical interventions like percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) may be required.
Cardiac rehabilitation supports recovery and health monitoring. While regular follow-up ensures effective treatment management.
Market Drivers
The global ischemic heart disease (IHD) treatment market is fueled by the rising prevalence of cardiovascular diseases, an aging population, and advancements in medical technology.
The growing incidence of IHD is the leading cause of death globally. Underscores the need for more treatment options, especially for older individuals at higher risk.
Innovations in diagnostics and therapies and government funding for research and awareness initiatives contribute to market growth.
Additionally, increased awareness of heart health and lifestyle changes driving disease prevalence further elevate the demand for effective IHD treatments.
Market Size
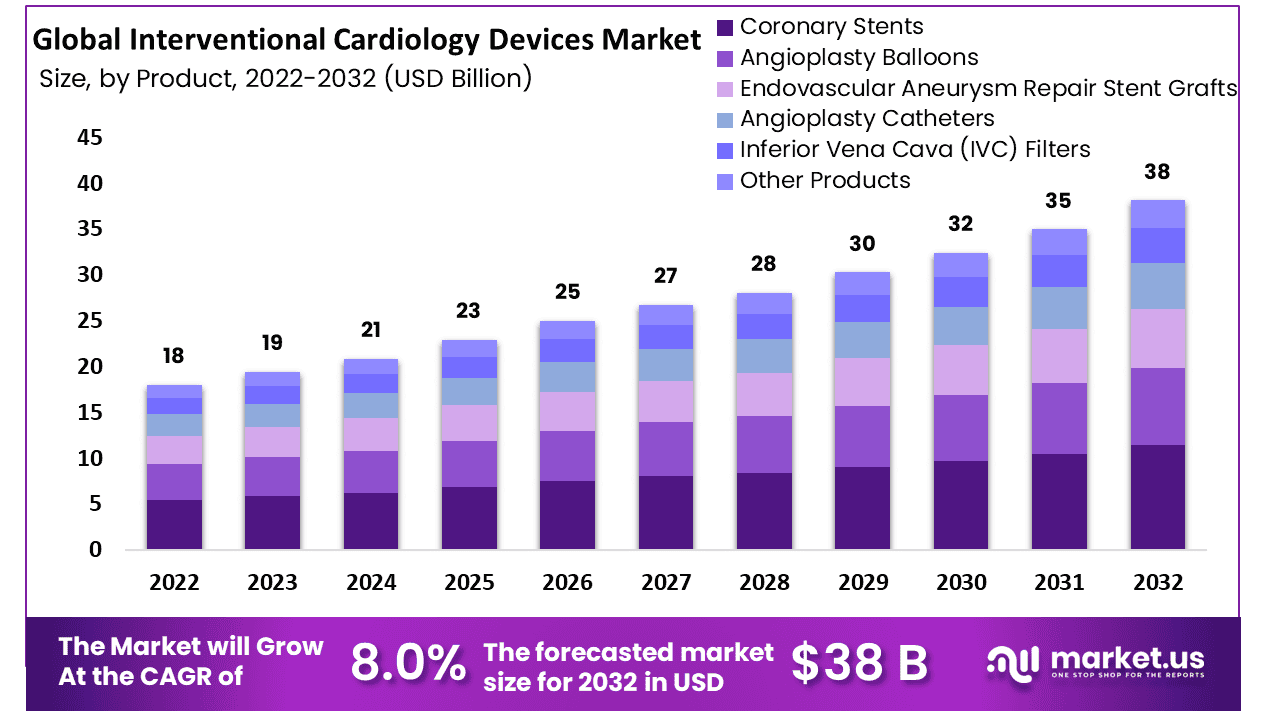
The global interventional cardiology devices market is expected to grow from USD 18 billion in 2022 to about USD 38 billion by 2032, with a CAGR of 8.0%.
List of Major Companies
These are the top ten companies operating in the Eating Disorder Treatment Market:
Abbott
Company Overview
| Establishment Year | 1888 |
| Headquarter | Green Oaks, Illinois, U.S. |
| Key Management | Robert B. Ford (CEO) |
| Revenue (US$ Bn) | $ 40.1 Billion (2023) |
| Headcount | ~ 114,000 (2023) |
| Website | http://abbott.com/ |
About Abbott
Abbott Laboratories has advanced the treatment of ischemic heart disease through its innovative medical devices and diagnostics.
Notably, the company offers the XIENCE Skypoint™ Stent System, which is designed to keep coronary arteries open and restore blood flow.
In April 2024, Abbott received FDA approval for the TriClip™ device. A minimally invasive option for tricuspid valve repair, expanding its structural heart intervention offerings.
Additionally, Abbott is engaged in clinical trials, such as the SPIRIT XLV Post Approval Study, to evaluate the safety and effectiveness of its coronary stent systems. Highlighting its commitment to enhancing treatment options and patient outcomes in this field.
Geographical Presence
Abbott Laboratories is a leading global healthcare company operating in over 160 countries, with around 70% of its revenue coming from international markets.
The company has a strong presence in North America, Europe, Asia-Pacific, Latin America, and the Middle East and Africa, offering tailored healthcare solutions to meet regional needs.
Abbott focuses on localized strategies by investing in manufacturing, supply chain, and research and development to enhance market responsiveness.
This extensive geographical reach and strategic approach position Abbott as a key player in advancing global healthcare and ensuring access to essential medical products and services worldwide.
Recent Developments
- In October 2024, Abbott terminated the sale of a 51% stake to The Searle Company Limited.
- In October 2024, Abbott collaborated with the Association of Physicians of India to address the health challenge of medication non-adherence.
Merck
Company Overview
| Establishment Year | 1891 |
| Headquarter | Rahway, New Jersey, U.S. |
| Key Management | Robert M. Davis (CEO) |
| Revenue (US$ Bn) | $ 60.1 B (2023) |
| Headcount | ~ 72,000 (2023) |
| Website | http://merck.com/ |
About Merck
Merck & Co., Inc. has been actively enhancing its cardiovascular portfolio, particularly in ischemic heart disease treatment.
In November 2021, Merck completed the acquisition of Acceleron Pharma Inc., integrating sotatercept. A novel therapy in Phase 3 development for pulmonary arterial hypertension, is in its pipeline.
In October 2024, Merck acquired Modifi Biosciences, a developer of experimental cancer therapies, in a deal potentially worth up to $1.3 billion.
These strategic acquisitions underscore Merck’s commitment to expanding its cardiovascular treatment options and addressing unmet medical needs in ischemic heart disease.
Geographical Presence
Merck & Co., Inc., or MSD outside North America, is a prominent global healthcare firm operating in over 120 countries and employing around 72,000 people.
In 2023, it generated $60.1 billion in revenue, with 47% from the U.S. The company’s revenue distribution includes $13.3 billion from Europe, the Middle East, and Africa, $6.8 billion from China, $3.2 billion from Japan, $3.1 billion from Asia Pacific, and $2.1 billion from Latin America.
Merck has research centers in Germany, the U.S., Japan, and China, as well as manufacturing sites in Ireland, Singapore, and Brazil. While actively expanding in emerging markets to boost future revenues.
Recent Developments
- In October 2024, Merck acquired Modifi Biosciences, gaining access to its experimental cancer therapies.
- In October 2024, Merck acquired CN201, an innovative bispecific antibody in clinical trials for B-cell-related diseases.
GSK
Company Overview
| Establishment Year | 2000 |
| Headquarter | London, England, UK |
| Key Management | Emma Walmsley (CEO) |
| Revenue (US$ Bn) | $ 37.7 Billion (2022) |
| Headcount | ~ 70,000 (2024) |
| Website | https://www.gsk.com/ |
About GSK
GSK plc is actively expanding its cardiovascular portfolio, particularly in ischemic heart disease treatments. In April 2022, the company announced its acquisition of Sierra Oncology for $1.9 billion, gaining momelotinib, a potential myelofibrosis treatment.
In May 2022, GSK planned to acquire Affinivax, a biopharmaceutical company focused on novel vaccines, for up to $3.3 billion, enhancing its vaccine capabilities.
Most recently, in January 2024, GSK agreed to acquire Aiolos Bio for up to $1.4 billion to bolster its respiratory pipeline with AIO-001, a phase II-ready antibody targeting the TSLP pathway.
These acquisitions highlight GSK’s commitment to strengthening its position in cardiovascular and respiratory markets.
Geographical Presence
GSK plc, based in Brentford, UK, is a prominent global pharmaceutical and biotechnology company with a diverse geographical presence.
In 2023, it generated around £6.6 billion in Europe, with major markets including the UK, Germany, and France.
The Americas accounted for £7.9 billion, primarily from the US and Canada, along with Brazil and Mexico. The Asia Pacific region contributed £5.5 billion, led by growth in China and India.
In comparison, Africa and the Middle East reported £2.3 billion. With key markets such as South Africa and Saudi Arabia. GSK’s extensive footprint highlights its strategic emphasis on both mature and emerging markets.
Recent Development
- In January 2024, GSK acquired Aiolos and its long-acting monoclonal antibody AIO-001, targeting thymic stromal lymphopoietin (TSLP). Which will enter phase II development for asthma and may also treat chronic rhinosinusitis with nasal polyps.
- In May 2022, GSK acquired Affinivax, a clinical-stage biopharmaceutical company based in Cambridge, Boston, Massachusetts
Novartis
Company Overview
| Establishment Year | 1996 |
| Headquarter | Basel, Switzerland |
| Key Management | Vasant Narasimhan (CEO) |
| Revenue (US$ Bn) | $ 45.4 Billion (2023) |
| Headcount | ~ 76,057 (2023) |
| Website | https://novartis.com/ |
About Novartis
Novartis AG has advanced its ischemic heart disease treatment portfolio through strategic acquisitions and innovative product developments.
In January 2020, Novartis completed the acquisition of The Medicines Company. Adding inclisiran—a first-in-class investigational cholesterol-lowering therapy—to its pipeline.
Inclisiran, marketed as Leqvio®, received U.S. FDA approval in December 2021 and has demonstrated effective and sustained LDL-C reduction in patients with atherosclerotic cardiovascular disease.
In June 2023, Novartis announced plans to acquire Chinook Therapeutics for up to $3.5 billion, aiming to bolster its renal pipeline and innovative medicines strategy.
These strategic initiatives underscore Novartis’s commitment to addressing cardiovascular diseases through both internal development and external acquisitions.
Geographical Presence
Novartis AG, headquartered in Basel, Switzerland, is a leading global pharmaceutical company with operations in over 140 countries.
In 2023, it reported total revenues of $45.5 billion, with significant contributions from the United States (33.88%), Germany (9.29%), Japan (5.76%), and China (5.29%).
The company has a robust presence in North America, particularly in states like Colorado and Massachusetts, and maintains strong operations in Europe and Asia-Pacific, with notable investments in China.
Novartis is actively expanding its footprint in emerging markets in Latin America and Africa. Allowing it to adapt to regional healthcare demands while balancing its risk profile across diverse markets.
Recent Development
- In September 2024, Novartis announced a collaboration with Generate: Biomedicines to create protein therapeutics across multiple disease areas.
- In September 2024, Novartis sold Worldwide Rights to DESFERAL (deferoxamine) to MITEM Pharma.
Johnson-n-Johnson
Company Overview
| Establishment Year | 1886 |
| Headquarter | New Brunswick, New Jersey, U.S. |
| Key Management | Joaquin Duato (CEO) |
| Revenue (US$ Bn) | $ 85.1 B (2023) |
| Headcount | ~ 134,400 (2023) |
| Website | https://www.jnj.com/ |
About Johnson & Johnson
Johnson & Johnson has significantly enhanced its position in the ischemic heart disease treatment sector through strategic acquisitions and product innovations.
In December 2022, the company acquired Abiomed, a leader in heart recovery technologies, expanding its cardiovascular offerings.
In May 2024, it acquired Shockwave Medical, known for its intravascular lithotripsy technology that treats calcified arterial lesions.
Furthermore, in October 2024, J&J acquired V-Wave Ltd., integrating its Ventura® Interatrial Shunt into its MedTech portfolio.
These acquisitions highlight Johnson & Johnson’s commitment to improving treatment options and patient outcomes for ischemic heart disease.
Geographical Presence
Johnson & Johnson (J&J) is a leading multinational corporation operating in over 60 countries. North America has significant manufacturing and R&D facilities in the United States and Canada.
In Europe, operations are centered in Ireland and the United Kingdom, with key sites in Cork and Slough. J&J is also prominent in the Asia-Pacific region, particularly in China, India, and Japan, and established its first Western pharmaceutical manufacturing site in China in 1985.
In Latin America, it has operations in Brazil and Mexico to improve access to medicines, and in Africa, it actively combats diseases like Ebola. J&J’s global distribution network supports its commitment to innovation and sustainability.
Recent Developments
- In October 2024, J&J announced its plans to invest US$2 billion in a new manufacturing facility in North Carolina, the US.
- In October 2024, J&J acquired V-Wave Ltd., enhancing Johnson & Johnson MedTech’s position in cardiovascular disease and its ability to address heart failure needs.
Bristol-Myers
Company Overview
| Establishment Year | 1887 |
| Headquarter | Princeton, New Jersey, U.S. |
| Key Management | Chris Boerner (CEO) |
| Revenue (US$ Bn) | $ 45.01 Billion (2023) |
| Headcount | ~ 34,000 (2023) |
| Website | http://bms.com/ |
About Bristol Myers Squibb
Bristol Myers Squibb has advanced its ischemic heart disease treatment portfolio through strategic acquisitions and innovative product developments.
In April 2022, the U.S. Food and Drug Administration approved Camzyos™ (mavacamten) for treating adults with symptomatic obstructive hypertrophic cardiomyopathy (HCM), marking a pivotal addition to their cardiovascular offerings.
In December 2023, the company announced the acquisition of RayzeBio, Inc., a leader in radiopharmaceuticals, for approximately $4.1 billion, aiming to enhance its oncology and cardiovascular treatment capabilities.
These strategic initiatives underscore Bristol Myers Squibb’s commitment to advancing treatment options for ischemic heart disease and improving patient outcomes.
Geographical Presence
Bristol Myers Squibb (BMS) is a prominent global biopharmaceutical company with a presence in North America, Europe, Asia-Pacific, and Latin America.
In the U.S., key facilities in New Jersey, Massachusetts, and California focus on research, biologics, and manufacturing, alongside operations in Montreal, Canada.
In Europe, BMS engages in R&D and commercial activities across countries like France, Switzerland, and the UK.
The Asia-Pacific operations in Japan and China emphasize manufacturing and clinical development, while Latin America includes a biologics facility in Puerto Rico. This extensive network enables BMS to develop and distribute innovative pharmaceuticals worldwide effectively.
Recent Developments
- In December 2023, Bristol Myers Squibb acquired RayzeBio, a clinical-stage company specializing in actinium-based radiopharmaceuticals and promising drug development programs.
- In April 2022, BMS announced FDA approval of Camzyos™ (mavacamten) capsules (2.5 mg, 5 mg, 10 mg, 15 mg) for adults with symptomatic NYHA class II-III obstructive hypertrophic cardiomyopathy to enhance functional capacity and symptoms.
Sanofi
Company Overview
| Establishment Year | 1973 |
| Headquarter | Paris, France |
| Key Management | Paul Hudson (CEO) |
| Revenue (US$ Bn) | $ 50.2 Billion (2022) |
| Headcount | ~ 86,088 (2023) |
| Website | http://www.sanofi.com/ |
About Sanofi
Sanofi S.A. has been actively enhancing its cardiovascular portfolio, particularly in ischemic heart disease treatment.
In April 2019, the U.S. Food and Drug Administration approved Praluent® (alirocumab) to reduce the risk of heart attack, stroke, and unstable angina requiring hospitalization in adults with established cardiovascular disease.
In May 2024, Sanofi completed the acquisition of Inhibrx, Inc., adding SAR447537 (formerly INBRX-101), a human recombinant protein designed to treat Alpha-1 Antitrypsin Deficiency, to its rare disease pipeline.
These strategic initiatives underscore Sanofi’s commitment to advancing treatment options for ischemic heart disease and improving patient outcomes.
Geographical Presence
Sanofi S.A., based in Paris, France, is a leading global healthcare company with a presence in over 100 countries. It employs around 45% of its workforce in Europe, particularly in France, Germany, and Italy.
In the Americas, its U.S. headquarters is in Bridgewater, New Jersey, with operations in Canada and Latin America.
The company also has manufacturing and research facilities in the Asia-Pacific region, including China, India, and Japan.
Recently, Sanofi announced a €1.1 billion investment in France to improve drug production, including a new facility in Vitry-sur-Seine, reflecting its commitment to innovation and global healthcare solutions.
Recent Developments
- In October 2024, Sanofi sold a 50% stake in its consumer health business, Opella, to Clayton Dubilier Rice.
- In May 2024, Sanofi acquired Inhibrx, Inc., adding SAR447537 (formerly INBRX-101) to its rare disease pipeline, reinforcing its commitment to developing best-in-class medicines.
Pfizer
Company Overview
| Establishment Year | 1849 |
| Headquarter | New York City, U.S. |
| Key Management | Albert Bourla (CEO) |
| Revenue (US$ Bn) | $ 58.5 Billion (2023) |
| Headcount | ~ 88,000 (2023) |
| Website | http://pfizer.com/ |
About Pfizer
Pfizer Inc. is contributing to treating ischemic heart disease through cardiovascular medications and partnerships.
The company offers Norvasc® (amlodipine besylate), a calcium channel blocker for hypertension and angina, which are risk factors for the disease.
In June 2021, Pfizer partnered with the American College of Cardiology and the Heart Rhythm Society to improve atrial fibrillation management in underserved communities.
Additionally, Pfizer is expanding its cardiovascular portfolio through acquisitions, notably its $43 billion purchase of Seagen Inc. in December 2023.
These efforts demonstrate Pfizer’s commitment to enhancing treatment options and patient outcomes for ischemic heart disease.
Geographical Presence
Pfizer Inc., a leading global pharmaceutical and biotechnology corporation, operates extensively across four primary regions: The United States, Developed Europe, Developed Rest of the World, and Emerging Markets. The United States accounts for approximately 46% of Pfizer’s total sales.
At the same time, Developed Europe includes key markets such as the UK, Germany, and France, where substantial investments in research and development are made.
The Emerging Markets, including China, India, and Brazil, are critical for growth, with China being one of Pfizer’s largest single markets.
Additionally, Pfizer maintains manufacturing and research facilities in various countries, enhancing its global supply chain and R&D capabilities ultimately ensuring the availability of its products to patients worldwide.
Recent Development
- In December 2023, Pfizer’s acquisition of Seagen Inc. expanded its Oncology pipeline to 60 programs, including ADCs, small molecules, bispecifics, and immunotherapies.
- In June 2021, BMS, the American College of Cardiology, and the Heart Rhythm Society launched a project to improve atrial fibrillation management in underserved communities.
Teva-Pharmaceutical
Company Overview
| Establishment Year | 1901 |
| Headquarter | Tel Aviv, Israel |
| Key Management | Richard Francis (CEO) |
| Revenue (US$ Bn) | $ 15.8 Billion (2023) |
| Headcount | ~ 37,851 (2023) |
| Website | http://www.tevapharm.com/ |
About Teva Pharmaceuticals
Teva Pharmaceuticals has been actively involved in the treatment of ischemic heart disease through its extensive portfolio of generic medications.
The company offers generic versions of essential cardiovascular drugs, including pravastatin sodium, a statin used to lower cholesterol and reduce the risk of coronary events.
In May 2023, Teva launched its “Pivot to Growth” strategy, aiming to expand its innovative medicines portfolio and focus on core therapeutic areas with first-in-class and best-in-class opportunities.
This strategic initiative underscores Teva’s commitment to advancing treatment options for cardiovascular diseases and improving patient outcomes.
Geographical Presence
Teva Pharmaceutical Industries Ltd., based in Israel, is a prominent global pharmaceutical company, offering over 3,600 medicines and producing approximately 76 billion tablets and capsules each year.
Employing around 37,000 people, Teva operates in 58 markets, reaching about 200 million individuals daily.
In North America, it is a key player in the generics sector, while in Europe, it serves over 30 countries and significantly contributes to healthcare savings.
Additionally, Teva is present in 25 international markets, including Latin America and Asia-Pacific. The company boasts more than 53 manufacturing facilities and 27 research and development sites worldwide, underscoring its commitment to accessible and affordable healthcare.
Recent Developments
- In October 2024, Teva Pharmaceuticals introduced a generic version of Sandostatin LAR Depot in the US.
- In October 2024, Teva and Alvotech received approval for SELARSDI (ustekinumab-aekn) from the US FDA.
AbbVie
Company Overview
| Establishment Year | 2012 |
| Headquarter | North Chicago, Illinois, United States |
| Key Management | Richard A. Gonzalez (CEO) |
| Revenue (US$ Bn) | $ 50.3 Billion (2023) |
| Headcount | ~ 50,000 (2023) |
| Website | http://abbvie.com/ |
About AbbVie
AbbVie’s recent acquisitions in neuroscience underscore its commitment to mental health, particularly regarding psychiatric conditions relevant to eating disorder treatment.
In 2023, AbbVie acquired Cerevel Therapeutics for $8.7 billion, enhancing its portfolio with assets targeting schizophrenia, Parkinson’s disease, and major depressive disorder, including emraclidine for schizophrenia and tavapadon for Parkinson’s.
Additionally, the acquisition of ImmunoGen provided access to Elahere, an antibody-drug conjugate for ovarian cancer.
These investments reflect AbbVie’s strategy to expand its neuroscience and psychiatric offerings. Which may have broader implications for managing mental health. Particularly for patients with eating disorders and comorbid conditions such as anxiety and depression.
Geographical Presence
AbbVie Inc. is a prominent global biopharmaceutical company with a geographical presence in over 70 countries across five major regions: North America (United States and Canada), Europe (Austria, Belgium, France, Germany, Italy, Netherlands, Spain, Sweden, Switzerland, and the United Kingdom), Asia Pacific (Australia, China, Hong Kong, Japan, Korea, Malaysia, New Zealand, Singapore, and Taiwan), Latin America/Caribbean (Argentina, Brazil, Chile, Colombia, Mexico, Puerto Rico, and Uruguay), and the Middle East/Africa (Algeria, Israel, Lebanon, Saudi Arabia, South Africa, Tunisia, and the United Arab Emirates).
This extensive footprint enables AbbVie to address diverse healthcare needs across global markets effectively.
Recent Developments
- In October 2024, it agreed to acquire Aliada Therapeutics for US$1.4 billion.
- In October 2024, AbbVie received FDA approval for VYALEV, a 24-hour infusion therapy for motor fluctuations in advanced Parkinson’s disease.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



