Table of Contents
Deep Brain Stimulation Device Market Overview
Deep Brain Stimulation (DBS) involves implanting a device that sends electrical impulses to targeted brain areas to treat movement disorders like Parkinson’s disease, essential tremor, and dystonia.
The system includes a pulse generator, electrodes in the brain, and a connecting extension. DBS modulates abnormal brain activity to reduce symptoms such as tremors and rigidity.
While it offers customizable and reversible relief, it carries risks like surgical complications and side effects, including mood or speech changes.
Advances such as adaptive and closed-loop DBS systems aim to improve treatment outcomes and minimize side effects.
Market Drivers
The global Deep Brain Stimulation (DBS) device market is driven by the rising prevalence of neurological disorders like Parkinson’s disease, essential tremor, and dystonia, especially with an aging population.
Technological advancements, such as MRI-compatible systems and rechargeable batteries, have made DBS devices more effective and patient-friendly.
The growing preference for minimally invasive procedures, increased healthcare spending, and government support for neurological research further boost market growth.
These factors, along with innovative treatments like the Picostim DBS system, contribute to the expanding adoption of DBS devices for treating movement disorders.
Market Size
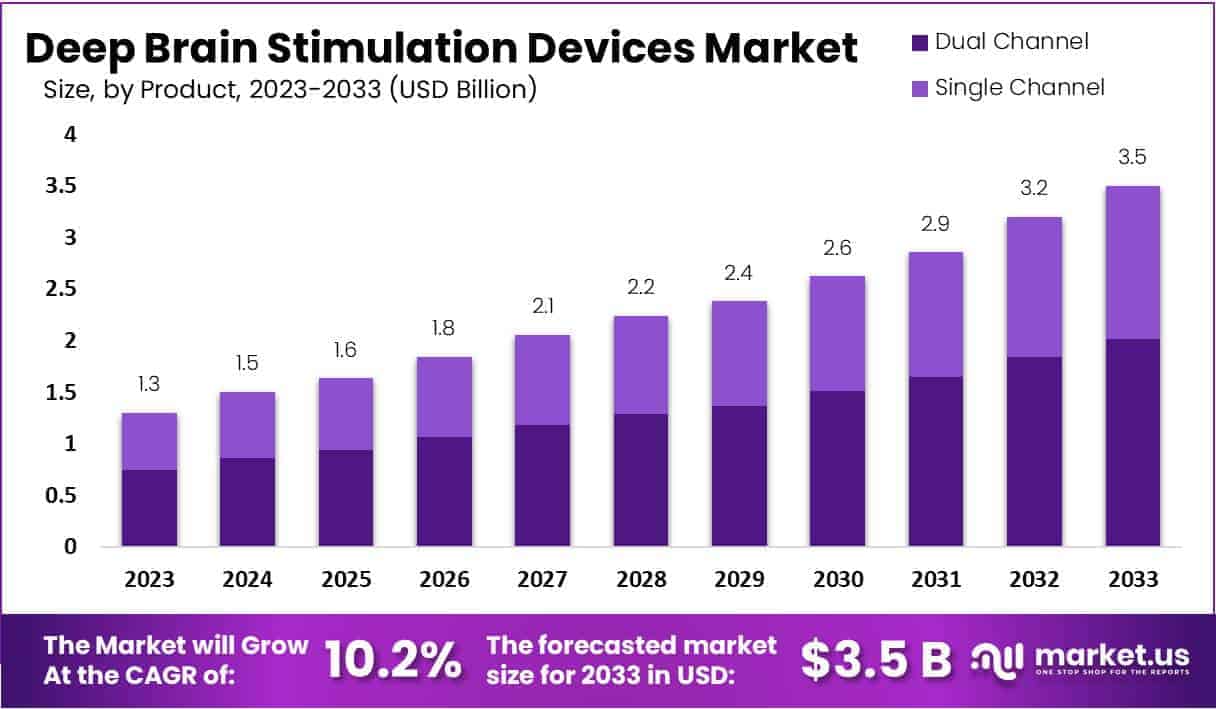
The Deep Brain Stimulation Devices Market is projected to grow from USD 1.3 billion in 2023 to USD 3.5 billion by 2033, with a CAGR of 10.2%.
List of Major Companies
These are the top ten companies operating in the Deep Brain Stimulation Device Market:
- Abbott
- Medtronic
- Boston-Scientific
- Neuropace
- Synapse-Biomedical
- Aleva-Neurotherapeutics
- Nexstim
- Jude-Medical
- NeuroSigma
- Synteract
Abbott
Company Overview
| Establishment Year | 1888 |
| Headquarter | Green Oaks, Illinois, U.S. |
| Key Management | Robert B. Ford (CEO) |
| Revenue (US$ Bn) | $ 40.1 Billion (2023) |
| Headcount | ~ 114,000 (2023) |
| Website | http://www.abbott.com/ |
About Abbott Laboratories
Abbott Laboratories has strengthened its position in the deep brain stimulation (DBS) device market, focusing on innovative solutions for movement disorders like Parkinson’s disease.
In January 2024, the company gained U.S. FDA approval for the Liberta RC™ DBS system, the world’s smallest rechargeable DBS device with remote programming and only ten recharges needed annually. This reflects Abbott’s commitment to improving patient outcomes through technological advances.
In September 2023, Abbott also acquired Bigfoot Biomedical, expanding its diabetes care portfolio and aligning with its strategy to integrate advanced technologies across its medical devices.
Geographical Presence
Abbott Laboratories operates in over 160 countries, with around 70% of its revenue generated outside the United States.
The company has a significant presence across various regions, including Asia Pacific (India, China, Japan, Indonesia, and others), Europe (Germany, France, Italy, the UK, and more), the Middle East and Africa (Saudi Arabia, South Africa, Israel), and the Americas (United States, Canada, Latin America, and Brazil).
This extensive geographical presence enables Abbott to address diverse healthcare needs and tailor its products and services to local markets.
Recent Developments
- In October 2024, Abbott’s TEAM-HF trial aimed to improve heart failure outcomes by enrolling 850 patients across 75 sites, using the CardioMEMS™ HF System to identify those who may benefit from the HeartMate 3™ LVAD earlier.
- In January 2024, Abbott introduced the FDA-approved Liberta RC™ DBS system. It is the smallest rechargeable device with remote programming, requiring only ten recharges annually for movement disorder treatment.
Medtronic
Company Overview
| Establishment Year | 1949 |
| Headquarter | Dublin, Ireland |
| Key Management | Geoff Martha (CEO) |
| Revenue (US$ Bn) | $ 32.3 B (2023) |
| Headcount | ~ 95,000 (2023) |
| Website | http://www.medtronic.com/ |
About Medtronic
Medtronic plc remains a leader in the deep brain stimulation device (DBS) market, providing advanced solutions for neurological disorders like Parkinson’s disease.
In January 2024, the company gained U.S. FDA approval for the Percept™ RC neurostimulator, a rechargeable DBS system with BrainSense™ technology that allows clinicians to monitor brain signals for personalized therapy. This approval expands the Percept™ family and strengthens Medtronic’s DBS portfolio.
Additionally, in August 2022, Medtronic acquired Affera, Inc. for $1 billion, enhancing its cardiac ablation and neuromodulation capabilities. These moves highlight Medtronic’s commitment to innovation and improving patient outcomes.
Geographical Presence
Medtronic plc, based in Dublin, Ireland, operates in over 150 countries, with key regional hubs in the Americas, Europe, the Middle East, Africa (EMEA), and Asia Pacific.
Its operational headquarters are in Minneapolis, USA. The company has a notable presence in the U.S., Brazil, Mexico, Germany, France, and the UK and is expanding markets in Asia, including China, India, Japan, and Australia.
Focused on emerging markets, Medtronic is strengthening its global footprint through strategic partnerships and localized initiatives to meet the growing demand for medical technologies.
Recent Developments
- In January 2024, Medtronic announced FDA approval for its Percept™ RC DBS system, part of the Percept™ family with BrainSense™ technology and SenSight™ leads.
- In August 2022, Medtronic acquired Affera, Inc., expanding its cardiac ablation portfolio with a fully integrated mapping, navigation, and ablation solution.
Boston-Scientific
Company Overview
| Establishment Year | 1979 |
| Headquarter | Marlborough, Massachusetts, U.S. |
| Key Management | Michael F. Mahoney (CEO) |
| Revenue (US$ Bn) | $ 14.2 Billion (2023) |
| Headcount | ~ 48,000 (2023) |
| Website | http://www.bostonscientific.com/ |
About Boston Scientific
Boston Scientific has established a strong presence in the deep brain stimulation device (DBS) market, focusing on treatments for neurological disorders like Parkinson’s disease and essential tremors.
Its Vercise™ DBS Systems, including the Vercise Genus™ platform, feature Multiple Independent Current Control (MICC) technology for precise, customizable stimulation.
In January 2021, the company received FDA approval for the Vercise Genus™ system, which includes Bluetooth-enabled, rechargeable, and non-rechargeable implantable pulse generators for improved patient comfort.
Additionally, in January 2024, Boston Scientific announced the $3.7 billion acquisition of Axonics, Inc., expanding its neuromodulation portfolio and strengthening its position in the market. These moves highlight the company’s commitment to advancing DBS technology and enhancing patient care.
Geographical Presence
Boston Scientific Corporation, headquartered in Marlborough, Massachusetts, operates in over 125 countries, with a strong presence across North America, Europe, the Middle East, Africa, Asia Pacific, and Latin America.
The company has regional headquarters in the U.S., France, Singapore, and Brazil, and its manufacturing facilities are located in locations such as California, Indiana, Ireland, Malaysia, Costa Rica, and Puerto Rico.
Additionally, it maintains research and development centers in the U.S., Poland, Japan, China, India, and other global hubs.
This extensive geographical presence enables Boston Scientific to deliver innovative medical solutions worldwide effectively.
Recent Development
- In November 2024, Boston Scientific acquired Axonics, Inc., a company specializing in devices for urinary and bowel dysfunction.
- In January 2021, Boston Scientific’s fourth-generation Vercise Genus™ DBS System was approved by the FDA. It features Bluetooth-enabled, rechargeable, and non-rechargeable IPGs with Cartesia™ Directional Leads for optimal symptom relief and MRI compatibility.
Neuropace
Company Overview
| Establishment Year | 1997 |
| Headquarter | Mountain View, California, United States |
| Key Management | Joel Becker (CEO) |
| Revenue (US$ Bn) | $ 65.4 Million (2023) |
| Headcount | ~ 171 (2023) |
| Website | https://www.neuropace.com/ |
About NeuroPace
NeuroPace, Inc. focuses on neuromodulation with its FDA-approved RNS® System, designed to treat drug-resistant focal epilepsy by monitoring and responding to abnormal brain activity.
While it does not offer traditional deep brain stimulation devices, the RNS System marks a key innovation in neuromodulation.
In November 2024, the company reported a 28% revenue increase to $21.1 million for Q3, driven by higher RNS System sales.
Additionally, NeuroPace submitted positive three-year data from its ongoing Post-Approval Study, highlighting its commitment to advancing treatments and improving patient outcomes.
Geographical Presence
NeuroPace, Inc., based in Mountain View, California, is a medical device company specializing in the development of implantable devices for treating neurological disorders, particularly drug-resistant epilepsy.
Its flagship product, the RNS® System, is approved for use in the United States, where the company primarily operates and markets its devices to hospitals.
As of now, NeuroPace’s geographical presence is limited to the U.S., with no public information indicating international regulatory approvals or marketing efforts.
Recent Development
- In November 2024, NeuroPace submitted three-year safety and effectiveness data to the FDA from its RNS System Post-Approval Study in adults with drug-resistant focal epilepsy.
Synapse-Biomedical
Company Overview
| Establishment Year | 2002 |
| Headquarter | St. Oberlin, Ohio, U.S. |
| Key Management | Anthony R. Ignagni (CEO) |
| Revenue (US$ Bn) | $ 61 M (2023) |
| Headcount | ~ 45 (2023) |
| Website | https://www.synapsebiomedical.com/ |
About Synapse Biomedical
Synapse Biomedical Inc. specializes in neurostimulation devices, notably the NeuRx® Diaphragm Pacing System (DPS), which provides electrical stimulation to the diaphragm to assist patients with respiratory insufficiency, such as those with spinal cord injuries or amyotrophic lateral sclerosis (ALS).
In April 2023, Synapse Biomedical received premarket approval (PMA) from the U.S. Food and Drug Administration (FDA) for the NeuRx DPS, expanding its availability for patients with spinal cord injuries who rely on mechanical ventilation.
This approval enhances the company’s position in the neurostimulation market, reflecting its commitment to improving patient outcomes through innovative respiratory solutions.
Geographical Presence
Synapse Biomedical Inc., headquartered in Oberlin, Ohio, is a company focused on the development of neurostimulation products, such as the NeuRx Diaphragm Pacing System.
The company has a strong international presence, with an office in Engien Les Bains, France, and partnerships across several regions, including Australia, Brazil, Germany, Japan, Saudi Arabia, South Korea, Spain, Taiwan, and the UAE, among others.
These international collaborations support the global distribution and service of Synapse Biomedical’s products, ensuring widespread availability and customer support.
Recent Developments
- In April 2023, Synapse Biomedical announced FDA premarket approval (PMA) for the NeuRx® Diaphragm Pacing System, simplifying adoption for spinal cord injury patients on mechanical ventilation.
Aleva-Neurotherapeutics
Company Overview
| Establishment Year | 2008 |
| Headquarter | Lausanne, Switzerland |
| Key Management | André Mercanzini (CEO) |
| Revenue (US$ Bn) | $ 66.5 Million (2023) |
| Headcount | ~ 21 (2023) |
| Website | https://aleva-neuro.com/ |
About Aleva Neurotherapeutics
Aleva Neurotherapeutics S.A., a Swiss company, develops advanced deep brain stimulation (DBS) devices for neurological disorders like Parkinson’s disease and essential tremors. Its directSTIM™ DBS System features a directional lead for precise electrical stimulation to optimize symptom relief.
In September 2022, Aleva received CE approval for MRI use of the system, broadening its patient accessibility.
Additionally, in February 2022, the FDA approved an investigational study for the directSTIM™ system, paving the way for potential U.S. market entry. These milestones highlight Aleva’s commitment to advancing DBS technology and expanding globally.
Geographical Presence
Aleva Neurotherapeutics S.A., headquartered in Lausanne, Switzerland, specializes in developing advanced Deep Brain Stimulation (DBS) systems for neurological disorders.
The company has received CE marking for its directSTIM™ DBS System, enabling its use in European Union countries, and has also obtained MRI approval for full-body imaging.
Aleva has established partnerships with leading neurological clinics in Europe and aims to expand its reach into other global markets, although specific details about its presence outside Europe are limited.
Recent Developments
- In September 2022, Aleva Neurotherapeutics received CE approval for MRI labeling of its directSTIM™ DBS System, enabling full-body MRI use. The system delivers targeted brain stimulation to treat Parkinson’s Disease, offering optimal relief and fewer side effects.
- In February 2022, Aleva Neurotherapeutics received FDA approval for an IDE study of its directSTIM DBS system for neurological conditions like Parkinson’s disease.
Nexstim
Company Overview
| Establishment Year | 2000 |
| Headquarter | Helsinki, Finland |
| Key Management | Mikko Karvinen (CEO) |
| Revenue (US$ Bn) | $ 2.7 Million (2024) |
| Headcount | ~ 37 (2023) |
| Website | https://www.nexstim.com/ |
About Nexstim
Nexstim, a Finnish medical technology company, specializes in non-invasive brain stimulation, particularly through its Navigated Brain Stimulation (NBS) and Navigated Brain Therapy (NBT®) systems.
Its NBS System 5 is FDA-cleared and CE-marked for pre-surgical mapping of the motor and speech cortices, aiding in neurosurgical planning.
In April 2023, Nexstim launched the NBS 6 system in the United States, enhancing usability and expanding its application in treating major depressive disorder (MDD).
Additionally, in October 2024, Nexstim gained an import license for the NBS System 5 in India, marking a significant step in its global expansion strategy.
These developments underscore Nexstim’s commitment to advancing non-invasive neuromodulation technologies and broadening their accessibility worldwide.
Geographical Presence
Nexstim Plc, headquartered in Helsinki, Finland, specializes in non-invasive brain stimulation technologies, with a focus on its SmartFocus® system that utilizes navigated transcranial magnetic stimulation (nTMS).
The company operates through subsidiaries in the United States and Germany, and it has a presence in Turkey and France, with distribution across North America, Europe, and Asia.
Nexstim’s network of partner clinics is concentrated in the U.S. and Europe, expanding access to its diagnostic and therapeutic systems. In 2024, the company expanded its reach by receiving an import license for its NBS System 5 in India, further extending its global footprint.
Recent Developments
- In November 2024, Nexstim announced that a long-time customer of a university hospital in the U.S. had placed an order for an NBS 5+ system.
- In April 2023, Nexstim launched the NBS 6, a new generation of navigated transcranial magnetic stimulation (nTMS) software, in the United States.
Jude-Medical
Company Overview
| Establishment Year | 1976 |
| Headquarter | Little Canada, Minnesota, U.S. |
| Key Management | Michael Rousseau (CEO) |
| Revenue (US$ Bn) | $ 3.7 Billion (2023) |
| Headcount | ~ 18,000 (2023) |
| Website | http://www.sjm.com/ |
About St. Jude Medical
St. Jude Medical, Inc., a prominent medical device company, has made significant contributions to the deep brain stimulation (DBS) sector, particularly with the development of the Infinity™ DBS System.
This system, approved by the U.S. Food and Drug Administration (FDA) in 2016, features directional lead technology designed to provide targeted stimulation for patients with Parkinson’s disease and essential tremor.
In January 2017, Abbott Laboratories completed the acquisition of St. Jude Medical, integrating its DBS technologies into Abbott’s neuromodulation portfolio.
Post-acquisition, Abbott has continued to advance DBS innovations, including the development of the Infinity™ DBS System with directional leads and wireless iOS-based programming, enhancing patient outcomes and therapy management.
These developments underscore the company’s commitment to improving neuromodulation therapies for neurological disorders.
Geographical Presence
St. Jude Medical, Inc., acquired by Abbott Laboratories in 2017, had a significant global presence before the acquisition.
The company operates in over 100 countries, with strong footholds in the United States, Europe, Latin America, and the Asia-Pacific region, including key markets like China, Japan, and India.
St. Jude Medical maintained six technology centers globally, in locations such as Brussels, Beijing, Tokyo, Austin, St. Paul, and Sylmar, which were instrumental in research, development, and training. Following its integration into Abbott, the reach and impact of St. Jude’s medical devices were further expanded worldwide.
NeuroSigma
Company Overview
| Establishment Year | 2008 |
| Headquarter | Los Angeles, California, U.S. |
| Key Management | Colin P. Kealey (CEO) |
| Revenue (US$ Bn) | $ 3.0 Million (2023) |
| Headcount | ~ 30 (2023) |
| Website | http://www.neurosigma.com/ |
About NeuroSigma
NeuroSigma, Inc., a Los Angeles-based bioelectronic medical device company, has primarily focused on developing and commercializing Trigeminal Nerve Stimulation (TNS) technologies, notably the Monarch eTNS® System, which is the first non-drug treatment for pediatric ADHD cleared by the FDA.
While the company has explored deep brain stimulation (DBS) technologies, its recent developments have centered on TNS applications. In January 2024, NeuroSigma received FDA clearance for its second-generation Monarch eTNS System, enhancing its offerings in the neuromodulation market.
Additionally, in May 2024, the company was accepted into the 2024 cohort of the KidsX Accelerator Program, aiming to expand its reach in pediatric healthcare.
These advancements underscore NeuroSigma’s commitment to innovating non-invasive neuromodulation therapies, with a primary focus on TNS rather than DBS technologies.
Geographical Presence
NeuroSigma, Inc., headquartered in Los Angeles, specializes in bioelectronic medical devices for neurological and neuropsychiatric disorders.
Its flagship product, the Monarch eTNS® System, has received regulatory approvals across several regions. In the U.S., it is FDA-cleared for treating pediatric ADHD. The European Union holds CE marking for use in ADHD, drug-resistant epilepsy, and major depressive disorder.
The device was also approved by Singapore’s Health Sciences Authority in 2023. Furthermore, in the UK, a large clinical trial for ADHD treatment is underway under approval from the Medicines and Healthcare Products Regulatory Agency. These approvals reflect NeuroSigma’s growing international presence.
Recent Developments
- In January 2024, NeuroSigma announced FDA clearance for its second-generation Monarch eTNS System to treat pediatric ADHD.
- In October 2023, NeuroSigma announced HSA approval of the Monarch eTNS System for treating pediatric ADHD in patients aged 7-12 off prescription medications.
Synteract
Company Overview
| Establishment Year | 1986 |
| Headquarter | Carlsbad, California, United States |
| Key Management | Steve Powell (CEO) |
| Revenue (US$ Bn) | $ 135.6 Billion (2023) |
| Headcount | ~ 1,000 (2023) |
| Website | http://www.synteract.com |
About Synteract
Synteract, Inc., a contract research organization (CRO), specializes in providing comprehensive clinical trial services to biopharmaceutical companies, with a focus on emerging biopharma segments.
The company offers expertise across various therapeutic areas, including neuroscience, supporting the development of treatments for neurological disorders. In December 2020, Synteract was acquired by Syneos Health, enhancing its capabilities and expanding its global reach.
This acquisition has enabled Synteract to leverage Syneos Health’s integrated biopharmaceutical solutions, thereby strengthening its support for clients in the deep brain stimulation (DBS) devices sector.
Post-acquisition, Synteract continues to operate as a Syneos Health business unit, maintaining its focus on delivering tailored clinical development services to meet the unique needs of emerging biopharma clients.
Geographical Presence
Synteract, Inc., established in 1986 and headquartered in Carlsbad, California, is a full-service contract research organization (CRO) specializing in clinical trials across various therapeutic areas.
Before its acquisition by Syneos Health in December 2020, Synteract operated in over 60 countries, with a strong presence in North America, Europe, Asia-Pacific, and Africa. Key office locations included the United States, Canada, several European countries, Australia, China, India, Japan, South Korea, Taiwan, and South Africa.
Following the acquisition, Synteract continues to operate as a Syneos Health Business Unit, leveraging Syneos Health’s global infrastructure to expand its clinical trial support for emerging biopharma companies.
Recent Developments
- In December 2020, Syneos Health finalized the acquisition of Synteract, a prominent full-service contract research organization (CRO) specializing in the fast-growing emerging biopharma sector.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



