Table of Contents
Overview
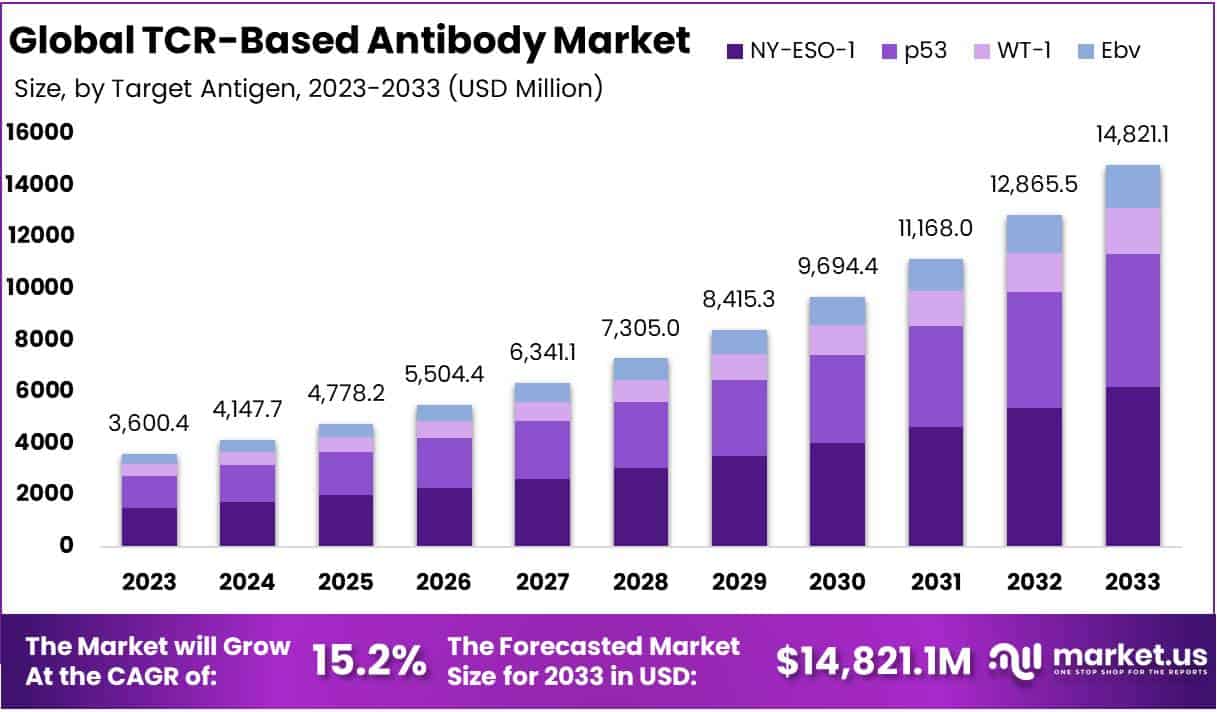
The global TCR-based antibody market is projected to reach USD 14,821.1 million by 2033, increasing from USD 3,600.4 million in 2023, at a CAGR of 15.2% during 2024–2033. The market’s expansion is driven by the growing ability of TCR-based antibodies to target intracellular antigens presented by MHC molecules. Unlike conventional antibodies limited to surface antigens, these therapies access previously untreatable targets. This scientific advancement broadens their therapeutic range and strengthens their position as next-generation immunotherapies. The increasing focus on immune-based treatments supports a positive growth outlook for this market.
Progress in antibody engineering and TCR design has enhanced the precision, affinity, and safety of these molecules. Continuous innovation in bispecific formats, affinity maturation, and humanization techniques has improved therapeutic outcomes. These advancements have transitioned TCR-based antibodies from research laboratories into early clinical trials. Moreover, their compatibility with precision medicine enables tailored treatment based on individual tumor profiles. This technological evolution ensures sustained scientific interest and commercial investment, creating a strong foundation for long-term market development.
The growing number of TCR-based antibodies entering clinical and preclinical stages demonstrates increasing industry confidence. Successful early-stage results have encouraged new entrants and research programs, expanding the innovation ecosystem. While oncology remains the primary therapeutic area, researchers are exploring autoimmune and infectious disease applications. The ability of these antibodies to modulate immune responses precisely opens new therapeutic possibilities beyond cancer. Such diversification is expected to enhance the commercial viability and broaden the future clinical impact of TCR-based antibody platforms.
The market benefits from collaborations among biotechnology companies, pharmaceutical developers, and research institutions. These partnerships accelerate discovery and enable integration with other immunotherapies, including checkpoint inhibitors and cell therapies. Regulatory agencies are increasingly supportive of novel immunotherapies addressing unmet medical needs. Clearer guidelines for safety, efficacy, and biomarker validation are expediting clinical translation. Together, these collaborations and regulatory initiatives provide a stable framework for accelerated innovation and commercialization of TCR-based antibody therapeutics.
The TCR-based antibody market is evolving rapidly through scientific innovation, regulatory support, and clinical expansion. Its capacity to recognize intracellular antigens and align with immuno-oncology and personalized medicine trends ensures sustained market growth. As research extends into diverse disease areas and partnerships strengthen development pipelines, the sector is positioned to become a key driver in next-generation antibody therapeutics. The convergence of technology, precision targeting, and supportive policies marks a transformative phase for this global market.
Key Takeaways
- The TCR-Based Antibody Market is projected to reach USD 14,821.1 million by 2033, growing at a CAGR of 15.2% from 2024–2033.
- NY-ESO-1 emerged as the leading target antigen in 2023, accounting for 41.7% of the market, followed by p53, WT-1, and EBV.
- Bladder cancer dominated the indication segment with over 43% market share in 2023, trailed by multiple myeloma and ovarian cancer applications.
- Hospitals held the largest end-user share at 42% in 2023, with specialized clinics, pharma and biotech labs, and gene therapy centers following.
- Rising global cancer deaths exceeding 10 million annually and 50 million Americans with autoimmune diseases are driving strong market demand.
- Market expansion is restrained by high drug development costs, averaging over $2.6 billion, which limit affordability and accessibility.
- Advances in genomic sequencing are fostering innovation, as cancer cases are expected to increase by 70% over the next twenty years.
- Collaboration activities in the biopharma industry have surged by 30%, accelerating R&D efficiency and reducing financial risks for novel therapies.
- North America maintained dominance in 2023 with a 36% market share, followed by Europe and the rapidly growing Asia-Pacific region.
Regional Analysis
In 2023, North America dominated the global TCR-based antibody market, holding over 36% share with a market value of USD 1,296.1 million. This leadership is supported by strong healthcare infrastructure and high investments in biotechnology and pharmaceutical research. The United States remains the key driver due to favorable regulations and R&D funding. Leading market players are concentrated in the region, which enhances innovation and accelerates product development, establishing North America as the central hub for TCR-based antibody advancements.
Europe ranked as the second-largest market, driven by advanced healthcare systems and growing interest in personalized medicine. Supportive government initiatives and research incentives have strengthened the region’s position. Countries such as Germany, the United Kingdom, and France lead the market due to active collaborations between research institutions and biopharmaceutical companies. The European Medicines Agency (EMA) has also promoted the approval of innovative therapies, fostering steady market expansion and positioning Europe as a competitive region in TCR-based antibody research.
The Asia-Pacific region is emerging as a fast-growing market for TCR-based antibodies. China, Japan, and South Korea are leading this expansion due to rising healthcare spending and a growing focus on advanced treatments. The increasing incidence of cancer and chronic diseases has encouraged demand for novel therapies. Regulatory reforms in several countries have improved the approval process for biologics. Strategic alliances between global biotech firms and local companies are enabling technology transfer, strengthening domestic expertise, and supporting the localization of cutting-edge therapeutic solutions.
Latin America and the Middle East & Africa (MEA) represent smaller market shares but are showing gradual progress. Rising awareness of cancer immunotherapies and improving healthcare infrastructure support future growth. Governments in these regions are investing in healthcare development to increase access to modern treatments. However, challenges persist, including affordability concerns, limited specialized facilities, and slow regulatory adaptation. Despite these obstacles, continued investments and awareness programs are expected to boost opportunities for TCR-based antibody adoption in the coming years.
Segmentation Analysis
In 2023, the TCR-Based Antibody Market was led by the NY-ESO-1 target antigen segment, accounting for 41.7% of the market share. The segment’s dominance was driven by its strong potential in cancer immunotherapy. NY-ESO-1 is highly specific to cancer cells while absent in normal tissues, making it an ideal target for precision therapies. Alongside this, the p53 and WT-1 antigens gained attention due to their significant roles in tumor progression, representing vital targets for future TCR-based antibody therapy developments.
The market’s indication analysis revealed that the Bladder Cancer segment dominated with over a 43% market share in 2023. This leadership was attributed to the global increase in bladder cancer cases and the growing preference for targeted therapies. Multiple Myeloma and Ovarian Cancer also demonstrated strong growth, supported by active R&D in TCR-based therapeutics. These developments have resulted in several clinical trials and investments aimed at improving treatment efficacy and expanding therapeutic options for patients.
Among end users, hospitals accounted for more than 42% of the TCR-Based Antibody Market in 2023. Their dominance was due to their advanced infrastructure and capacity to manage complex therapeutic procedures. Specialized clinics followed, offering focused patient care and personalized treatment plans. Pharma and biotech laboratories played a critical role in driving innovation and research collaborations, while gene therapy centers represented a rapidly expanding niche, emphasizing the growing adoption of gene-editing techniques to enhance TCR-based therapy effectiveness.
Key Players Analysis
The TCR-Based Antibody Market is driven by key players contributing significantly to advancements in immunotherapy. Lion TCR leads innovation through its personalized T-cell receptor therapies. The company focuses on harnessing the body’s immune system to fight diseases effectively. GlaxoSmithKline (GSK) leverages its global R&D capabilities and strategic partnerships to enhance TCR-based antibody treatments. These organizations are setting new benchmarks in precision medicine by integrating scientific expertise with robust clinical research and development frameworks.
Adaptimmune Therapeutics PLC plays a vital role in the market with its specialization in TCR-engineered T-cell therapies for cancer. The company focuses on targeting solid tumors through advanced genetic engineering techniques. Celgene Corporation, now a subsidiary of Bristol Myers Squibb, adds biopharmaceutical excellence to the segment. Its work primarily emphasizes hematologic malignancies and solid tumor treatments. The collaboration between Adaptimmune and other oncology leaders continues to strengthen the research landscape and expand therapeutic possibilities.
Immunocore and Kuur Therapeutics Limited also contribute notably to the TCR-based antibody ecosystem. Immunocore is recognized for its proprietary TCR bispecific technology designed to redirect T cells against cancer cells. Kuur Therapeutics specializes in cell-based immunotherapies that combine innovation with clinical applicability. Both companies are instrumental in improving treatment outcomes for patients with challenging oncological conditions. Their continued research and strategic investments highlight the growing confidence in immune-based therapeutic solutions across global healthcare markets.
Additionally, emerging and established companies such as Kite Pharma, Takara Bio Inc., Ziopharm Oncology Inc., and Merck & Co. Inc. are expanding the market’s potential. Their contributions span from cutting-edge research to large-scale commercialization efforts. These organizations collaborate across regions to develop novel solutions in TCR technology. Collectively, they drive market growth through strategic alliances, clinical advancements, and diversified pipelines. Their combined efforts are accelerating innovation in precision medicine and reinforcing the global competitiveness of TCR-based therapies.
Market Key Players
- Lion TCR
- GlaxoSmithKline
- Adaptimmune Therapeutics PLC
- Celgene Corporation
- Immunocore
- Kuur Therapeutics Limited
- Lion TCR Pte. Ltd.
- Kite Pharma
- Takara Bio Inc.
- Ziopharm Oncology Inc.
- Merck & Co. Inc.
Conclusion
The TCR-based antibody market is advancing rapidly, supported by scientific innovation and rising interest in immune-based therapies. The ability of these antibodies to target previously inaccessible intracellular antigens has made them a promising option in modern medicine. Continuous progress in antibody engineering, clinical research, and global collaborations is enhancing treatment accuracy and expanding therapeutic potential. As more products enter clinical trials and regulatory support increases, the market is expected to see steady growth. The expanding role of precision medicine and ongoing technological improvements ensure that TCR-based antibodies will remain at the forefront of next-generation immunotherapy development.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



