Table of Contents
Introduction
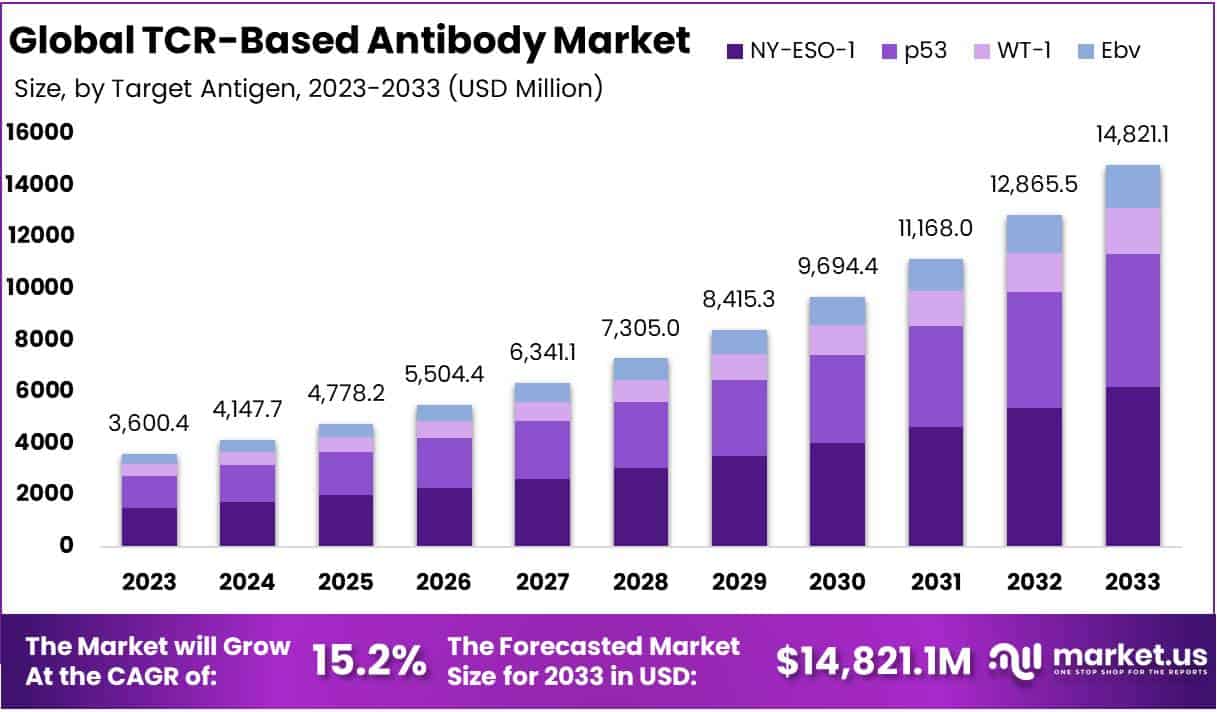
The Global TCR-Based Antibody Market is projected to reach approximately USD 14.82 billion by 2033, increasing from USD 3.6 billion in 2023. This growth reflects a robust compound annual growth rate (CAGR) of 15.2% during the forecast period from 2024 to 2033. The expansion of this market is driven by advancements in TCR-T therapies, clinical developments, and increasing research efforts.
Advancements in TCR-engineered T-cell (TCR-T) therapies have contributed significantly to market growth. Unlike CAR-T therapies, TCR-T treatments can target peptides derived from intracellular proteins, enabling them to address a broader range of tumor antigens. This capability makes TCR-T therapies highly effective against both blood-related cancers and solid tumors. Ongoing innovations in TCR design are improving treatment outcomes and reducing side effects, boosting their adoption in clinical practices.
Clinical developments have further accelerated growth in the TCR-based antibody market. The approval of afamitresgene autoleucel (Tecelra), designed to target MAGE-A4 for advanced synovial sarcoma, highlights the rising clinical acceptance of TCR-T therapies. Additionally, promising trial results for treatments targeting antigens such as NY-ESO-1 in soft tissue sarcoma and liposarcoma have reinforced the potential of TCR-based treatments in various cancer types.
The discovery of new tumor antigens has broadened the scope of TCR-based therapies. Researchers are exploring targets such as Laminin subunit gamma 2 (LAMC2) in pancreatic cancer and Claudin-6 (CLDN6) in ovarian cancer. These advancements are expanding the range of cancers that can be treated with TCR-based therapies, increasing market opportunities.
Research and development initiatives play a crucial role in enhancing TCR therapies. Efforts to improve TCR specificity, optimize treatment design, and explore combination therapies are ongoing. These strategies aim to overcome challenges such as tumor heterogeneity and immune evasion, ensuring better treatment success rates. With continued research efforts, TCR-based therapies are expected to witness further improvements, supporting sustained market growth.
Key Takeaways
- The TCR-Based Antibody Market is projected to reach USD 14.82 billion by 2033, expanding at a CAGR of 15.2% from 2024-2033.
- NY-ESO-1 held a 41.7% market share in 2023, outperforming other segments such as p53, WT-1, and EBV.
- Bladder cancer dominated with over 43% market share in 2023, followed by multiple myeloma and ovarian cancer treatments.
- Hospitals led with a 42% share in 2023, followed by specialized clinics, pharma labs, and gene therapy centers.
- Rising global cancer deaths and over 50 million Americans with autoimmune diseases continue to fuel market demand.
- High drug development costs, averaging over USD 2.6 billion, limit accessibility and slow market growth.
- Genomic sequencing advancements boost innovation, with cancer cases expected to increase by 70% in the next two decades.
- Biopharma collaborations increased by 30%, accelerating research, development, and risk-sharing for innovative therapies.
- North America led with 36% market share in 2023, followed by Europe and Asia-Pacific regions.
Emerging Trends
- Advancements in Cancer Immunotherapy: TCRm antibodies are emerging as a promising innovation in cancer immunotherapy. These antibodies target intracellular antigens, which expands the range of potential tumor targets. Unlike traditional therapies that focus mainly on surface proteins, TCRm antibodies can recognize proteins inside cancer cells. This advancement offers new possibilities for treating cancers that were previously difficult to target. Preclinical studies have shown positive results, demonstrating the potential of TCRm antibodies in improving cancer treatment outcomes. As research advances, these antibodies may become a key tool in precision oncology.
- Development of Bispecific TCR-Based Therapies: Innovations in TCR-based therapies have led to the creation of bispecific treatments such as ImmTACs (Immune mobilizing monoclonal T-cell receptors Against Cancer). These therapies combine engineered TCRs with immune-activating complexes. This design helps redirect cytotoxic T cells to identify and destroy cancer cells. ImmTACs offer a targeted approach, improving precision in cancer treatment. By engaging the body’s immune system directly, these therapies aim to enhance the effectiveness of cancer immunotherapy. Their ability to target a wider range of tumors makes them a promising option in oncology.
- Clinical Advancements and Regulatory Progress: The approval of afamitresgene autoleucel (Tecelra) by the U.S. FDA in August 2024 marked a major achievement for TCR-based therapies. This therapy is the first approved TCR gene therapy designed to treat synovial sarcoma. Tecelra’s approval demonstrates the potential of TCR-based treatments in managing rare and aggressive cancers. Its success may encourage further research and investment in TCR-based immunotherapies. As clinical trials expand, these therapies are expected to provide more targeted treatment options for various cancer types.
Use Cases
- Targeting Intracellular Tumor Antigens: TCRm antibodies are designed to recognize peptides from intracellular tumor antigens presented by MHC molecules. This ability allows TCR-based therapies to target a wider range of tumor-associated antigens that conventional antibodies may overlook. Unlike standard monoclonal antibodies that mainly target surface proteins, TCRm antibodies can access tumor markers hidden inside cells. This expanded targeting capability makes TCRm antibodies valuable in treating various cancers, especially those with limited treatment options. Their precision in identifying intracellular targets offers new possibilities in improving cancer immunotherapy outcomes.
- Combination Therapies: TCR-based therapies are showing promise when combined with other immunotherapies. These combination approaches aim to boost anti-tumor responses, particularly in cancers that are less responsive to current treatments. By pairing TCR-based antibodies with immune checkpoint inhibitors or cytokine therapies, researchers aim to enhance the immune system’s ability to detect and destroy cancer cells. This strategy is being actively studied in clinical trials, especially for challenging cancers such as melanoma and lung cancer. Combining treatments may improve patient outcomes and reduce the risk of resistance to single-agent therapies.
- Autoimmune Disease Treatment: TCR-targeted antibodies are also being explored for treating autoimmune diseases. These antibodies can selectively target and eliminate pathogenic T cells that contribute to immune system dysfunction. By removing these harmful cells, TCR-based therapies may help restore immune balance without broadly suppressing the entire immune system. This targeted approach has the potential to improve treatment outcomes in conditions such as rheumatoid arthritis, multiple sclerosis, and type 1 diabetes. Researchers are actively exploring TCR-based treatments to offer safer and more effective alternatives for managing autoimmune disorders.
Conclusion
The TCR-based antibody market is set to witness substantial growth driven by advancements in cancer immunotherapy, innovative treatment designs, and promising clinical developments. The ability of TCR-based therapies to target intracellular tumor antigens offers new possibilities for treating cancers with limited options. Additionally, combining TCR therapies with other immunotherapies is enhancing treatment outcomes. Research into autoimmune disease treatments using TCR-targeted antibodies is expanding their potential beyond oncology. As innovation continues and clinical trials show positive results, TCR-based therapies are expected to play a vital role in improving cancer care and autoimmune disease management. These factors collectively position the TCR-based antibody market for strong growth in the coming years.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



