Table of Contents
Overview
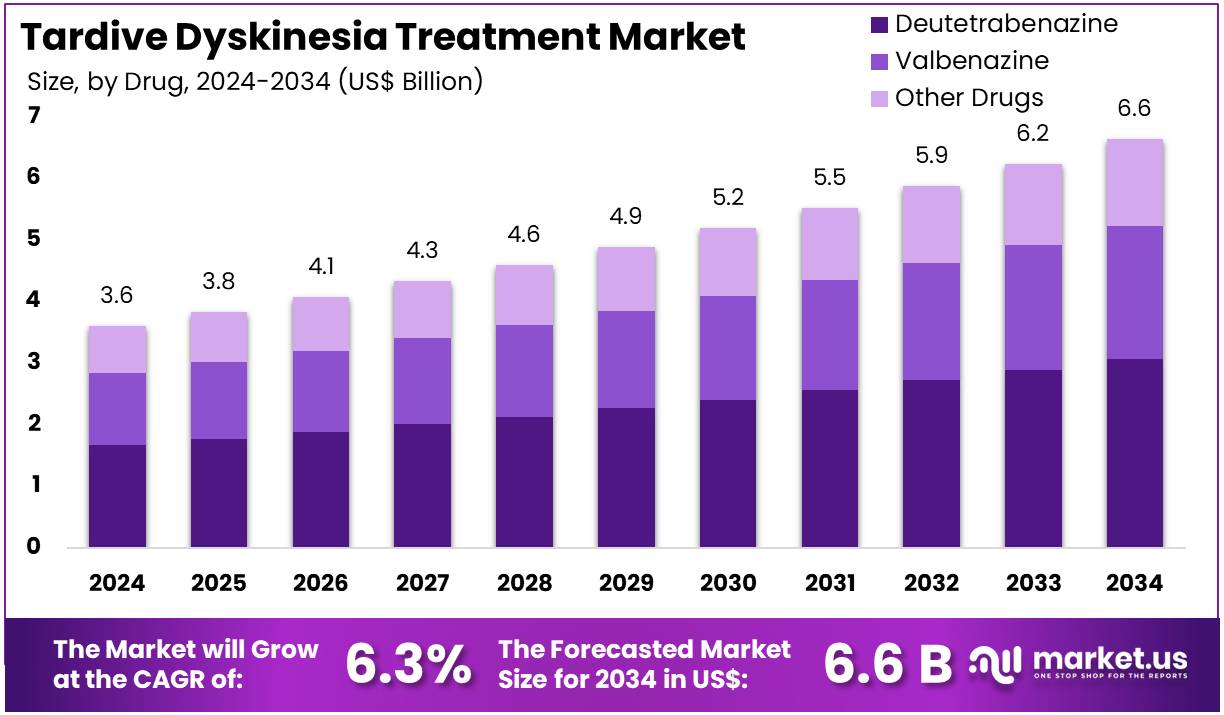
New York, NY – July 30, 2025 – The Tardive Dyskinesia Treatment Market size is expected to be worth around US$ 6.6 billion by 2034 from US$ 3.6 billion in 2024, growing at a CAGR of 6.3% during the forecast period 2025 to 2034.
The global tardive dyskinesia treatment market is experiencing consistent growth, fueled by increased prevalence of psychiatric disorders and long-term antipsychotic medication use. Tardive dyskinesia, a neurological disorder characterized by involuntary movements, is primarily induced by dopamine receptor-blocking agents used to manage conditions such as schizophrenia, bipolar disorder, and major depressive disorder. The treatment landscape is dominated by vesicular monoamine transporter 2 (VMAT2) inhibitors, including deutetrabenazine and valbenazine, which have shown clinical efficacy in reducing symptoms with fewer adverse effects.
Segmentation of the market includes drug type (VMAT2 inhibitors, benzodiazepines, others), distribution channel (hospital pharmacies, retail pharmacies, online pharmacies), and end-user (hospitals, specialty clinics, homecare). North America held the largest revenue share in 2023, driven by FDA approvals, structured reimbursement policies, and high healthcare awareness.
Emerging trends include the development of novel agents targeting underlying neurochemical pathways, increased research funding, and the integration of digital symptom tracking tools. Asia-Pacific is expected to register the fastest growth due to rising psychiatric diagnoses and improving access to neurology care. The market continues to evolve with increasing clinical trials and regulatory support for newer therapies.
Key Takeaways
- In 2024, the global tardive dyskinesia treatment market generated a revenue of US$ 3.6 billion. The market is projected to grow at a compound annual growth rate (CAGR) of 6.3%, reaching approximately US$ 6.6 billion by 2034.
- The market is segmented by drug type into deutetrabenazine, valbenazine, and other drugs. Among these, deutetrabenazine emerged as the leading segment in 2023, accounting for a market share of 46.3%, owing to its established clinical efficacy and regulatory approvals.
- Based on the distribution channel, the market is categorized into hospital pharmacies, retail pharmacies, and online pharmacies. In this segment, hospital pharmacies dominated with a significant share of 41.5%, supported by the preference for specialist consultations and institutional prescribing practices.
- Regionally, North America maintained its leading position in the global market, contributing to 39.6% of the total revenue in 2023. This dominance can be attributed to the region’s strong healthcare infrastructure, high prevalence of neuropsychiatric conditions, and early adoption of novel therapeutic agents.
Segmentation Analysis
- Drug Analysis: Deutetrabenazine accounted for 46.3% of the market share due to its targeted VMAT2 inhibition and demonstrated effectiveness in treating tardive dyskinesia. Its ability to modulate dopamine activity with fewer side effects has made it a preferred option among clinicians. Ongoing clinical research, expanding therapeutic indications, and rising provider confidence are expected to support its continued dominance. Increased patient awareness and growing adoption further reinforce deutetrabenazine’s strong position in the evolving treatment landscape.
- Distribution Channel Analysis: Hospital pharmacies held a 41.5% market share, driven by their central role in the diagnosis and management of movement disorders. Hospitals offer specialized care and infrastructure needed for early detection and treatment of tardive dyskinesia, particularly in aging populations. Improved insurance coverage and reimbursement policies for approved drugs enhance accessibility. The ability to provide end-to-end care, including diagnosis, medication dispensing, and follow-up, ensures hospital pharmacies remain a dominant distribution channel in this market.
Market Segments
By Drug
- Deutetrabenazine
- Valbenazine
- Other Drugs
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Regional Analysis
North America Leads the Global Market
North America held the largest revenue share of 39.6% in 2023, driven by proactive efforts to diagnose and manage tardive dyskinesia linked to long-term antipsychotic use. According to findings presented at the 2024 American Psychiatric Association Annual Meeting, the prevalence of tardive dyskinesia among U.S. antipsychotic users ranged between 94.1 and 127.4 per 1,000 users across commercial, Medicare, and Medicaid groups. This high prevalence has encouraged increased clinical focus and policy support to enhance treatment accessibility.
The U.S. Food and Drug Administration (FDA) continues to support innovation in this area, with recent approvals such as Austedo XR (deutetrabenazine extended-release) in May 2024, offering more convenient once-daily dosing. These regulatory advancements, coupled with expanded therapeutic options, are improving patient outcomes and access to care. The combination of increasing awareness, strong regulatory infrastructure, and a high diagnostic rate is expected to sustain North America’s leading position in the global market throughout the forecast period.
Asia Pacific Expected to Witness Fastest Growth
The Asia Pacific region is anticipated to register the highest CAGR during the forecast period, supported by growing awareness of mental health conditions and their associated complications. While regional prevalence data remains limited, the increasing use of antipsychotics and recognition of their long-term side effects have elevated the need for targeted therapies like those used in tardive dyskinesia.
Rapid improvements in healthcare infrastructure across countries such as India, China, and Southeast Asian nations are enhancing diagnostic and treatment capabilities. This evolving landscape enables earlier identification and management of drug-induced movement disorders. Additionally, rising public and private investment in psychiatric healthcare is contributing to the demand for effective therapeutic solutions.
Pharmaceutical companies are actively expanding their presence in Asia Pacific, focusing on the development and distribution of novel therapies. As awareness of tardive dyskinesia grows among both clinicians and patients, the region is expected to emerge as a key growth market for treatment solutions over the next decade.
Emerging Trends
- Dominance of VMAT2 Inhibitors: VMAT2 inhibitors have been established as the primary pharmacological approach following FDA approvals of valbenazine (Ingrezza) in April 2017 and deutetrabenazine (Austedo) in August 2017. These approvals marked the first medications specifically indicated for adults with tardive dyskinesia, shifting management from off-label agents to targeted therapies.
- Extended and Sprinkle Formulations to Enhance Adherence: Extended-release and sprinkle formulations have been introduced to improve patient convenience and adherence. In late 2017, an 80 mg capsule strength of valbenazine received FDA clearance, allowing flexible dosing without increasing pill burden. Such formulations are expected to support consistent daily dosing and reduce missed doses.
- Expansion of Clinical Research Pipelines: A growing number of interventional studies is underway, investigating novel compounds and delivery methods. Over 10 active trials are currently registered on ClinicalTrials.gov, evaluating new VMAT2 inhibitors, remote monitoring technologies, and neuromodulation strategies for TD management.
- Exploration of Neuromodulation Techniques: Non-drug approaches such as focused ultrasound (e.g., ExAblate Transcranial MRgFUS) and deep brain stimulation are being explored in early-phase trials. These techniques aim to modulate abnormal motor circuits directly and may offer therapeutic options for patients who do not respond to pharmacotherapy.
- Investigation of Antioxidant and Repurposed Agents: Research into repurposed antioxidants such as Ginkgo biloba extract (EGb-761) and vitamin B6 is ongoing. A 12-week trial in 157 patients demonstrated a 51.3% response rate (≥ 30% reduction in AIMS score) with 240 mg/day of Ginkgo biloba versus 5.1% with placebo, highlighting potential adjunctive benefits.
Use Cases
- Valbenazine for Chronic Symptom Control: Valbenazine has been shown to reduce abnormal involuntary movement scale (AIMS) scores by an average of 3.2 points at a 40 mg dose versus placebo over 6 weeks in pivotal trials. Once-daily dosing and a favorable side-effect profile have led to its adoption in routine TD management.
- Deutetrabenazine for Personalized Dosing: In a phase 3 trial of 298 participants, deutetrabenazine at 24 mg/day and 36 mg/day produced mean AIMS score improvements of 3.2 and 3.3 points, respectively, compared to 1.4 points with placebo. This dose-response data supports individualized dosing strategies based on efficacy and tolerability.
- Ginkgo Biloba as an Adjunct Therapy: Inpatients receiving 240 mg/day of EGb-761 exhibited a mean AIMS score decrease of 2.13 ± 1.75, compared to 0.10 ± 1.69 with placebo, and 51.3% achieved a ≥ 30% improvement at week 12. These results suggest a role for antioxidant supplementation alongside primary treatment.
- Remote Monitoring via Wearable Sensors: Feasibility studies (e.g., NCT06011408) are validating wearable devices for continuous monitoring of involuntary movements, with preliminary data indicating over 85% accuracy in detecting dyskinetic episodes. Such approaches promise objective symptom tracking outside clinic visits.
- Neuromodulation in Refractory Cases: Early-phase trials of ExAblate MRgFUS have enrolled participants to assess safety and efficacy in TD symptom reduction. While data remain preliminary, these interventions are being positioned for patients unresponsive to pharmacotherapy, with future results expected to guide clinical application.
Conclusion
The global tardive dyskinesia treatment market is poised for significant growth, driven by rising psychiatric disorder prevalence, increased antipsychotic use, and advancements in targeted therapies like VMAT2 inhibitors. Deutetrabenazine and valbenazine remain the preferred treatments, supported by robust clinical evidence and favorable regulatory approvals.
North America leads due to strong healthcare infrastructure, while Asia Pacific is expected to register the fastest growth owing to improved awareness and expanding healthcare systems. Emerging trends, including digital monitoring, neuromodulation, and adjunct antioxidant therapies, are reshaping the treatment landscape. Continued research and innovation will further enhance treatment outcomes and market expansion.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



