Table of Contents
Overview
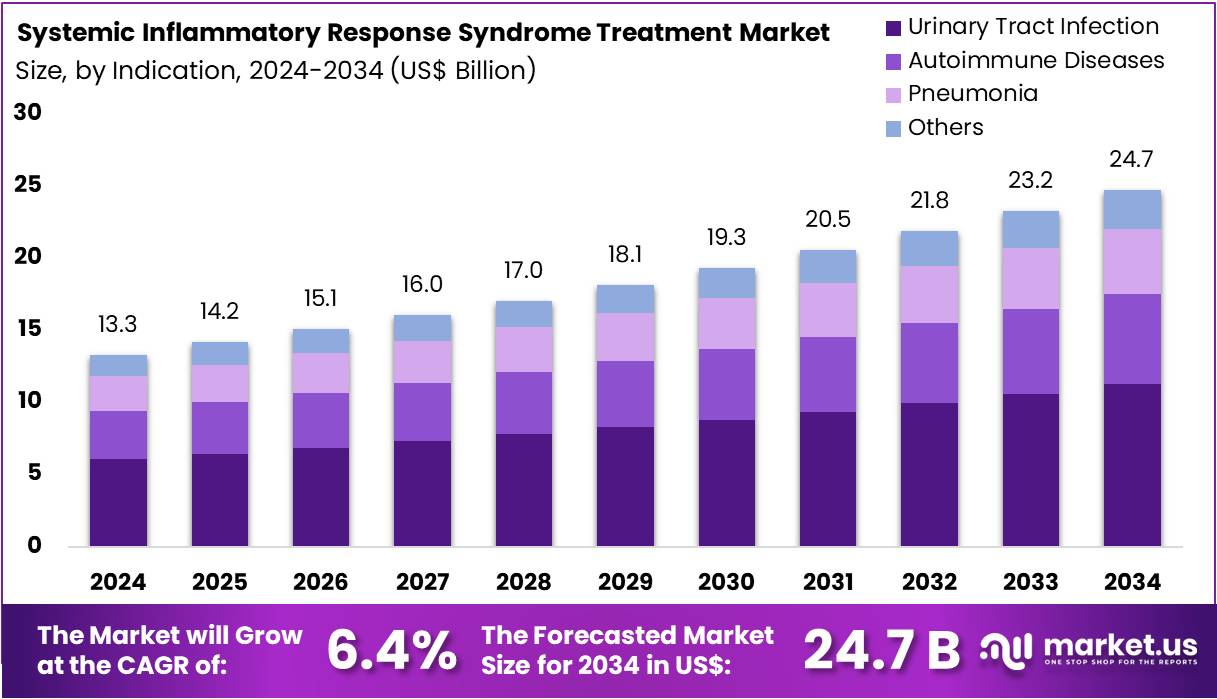
New York, NY – July 31, 2025 – The Systemic Inflammatory Response Syndrome Treatment Market size is expected to be worth around US$ 24.7 billion by 2034 from US$ 13.3 billion in 2024, growing at a CAGR of 6.4% during the forecast period 2025 to 2034.
The global market for Systemic Inflammatory Response Syndrome (SIRS) treatment is expected to witness stable growth over the forecast period, fueled by increasing incidence of sepsis, trauma-related inflammation, and post-surgical complications. SIRS is a critical medical condition characterized by systemic inflammation that may lead to multi-organ dysfunction if left untreated.
Treatment approaches primarily include supportive care, fluid resuscitation, oxygen therapy, vasopressors, corticosteroids, and immunomodulators. A growing focus on early diagnosis and the use of biomarkers such as procalcitonin and C-reactive protein has improved treatment outcomes. Hospitals and intensive care units remain the primary end-users, supported by a rising number of ICU admissions globally.
North America dominates the market due to well-established critical care infrastructure and favorable reimbursement policies, while Asia Pacific is expected to register significant growth, attributed to improving healthcare access and rising awareness of systemic inflammatory complications.
Emerging trends include the use of extracorporeal blood purification techniques and novel anti-inflammatory biologics under clinical trials. Additionally, ongoing research efforts are evaluating the role of precision medicine and AI-based decision support systems in critical care environments. The continued emphasis on managing hospital-acquired infections and reducing ICU mortality rates is anticipated to strengthen demand for effective SIRS treatment protocols across global healthcare systems.
Key Takeaways
- In 2024, the global market for systemic inflammatory response syndrome (SIRS) treatment was valued at US$ 13.3 billion, and it is projected to grow at a compound annual growth rate (CAGR) of 6.4%, reaching approximately US$ 24.7 billion by 2034.
- By indication, the market is segmented into urinary tract infection, autoimmune diseases, pneumonia, and others. Among these, urinary tract infections accounted for the largest share, contributing 45.6% of the total market value in 2023, driven by the high prevalence and increased risk of SIRS among affected patients.
- Based on end-user segmentation, the market includes hospitals, ambulatory surgical centers, specialty clinics, and others. Hospitals emerged as the dominant segment, holding a 55.7% share in 2023, owing to the high volume of critical care cases and access to advanced treatment infrastructure.
- Regionally, North America led the global SIRS treatment market, securing a 41.8% market share in 2023, supported by advanced healthcare systems, increasing ICU admissions, and early adoption of innovative critical care technologies.
Segmentation Analysis
- Indication Analysis: Urinary tract infection (UTI) accounted for 45.6% of the indication segment in the systemic inflammatory response syndrome (SIRS) treatment market. This dominance is attributed to the high global prevalence of UTIs, especially among women, the elderly, and those with chronic illnesses. Rising antimicrobial resistance and the need for early intervention to prevent complications such as sepsis are expected to fuel demand for advanced SIRS therapies targeting infection-induced systemic inflammation.
- End-User Analysis: Hospitals held the largest share of 55.7% in the SIRS treatment market by end-user in 2023. Their dominance stems from being the primary care setting for managing acute conditions such as sepsis, trauma, and infection-induced SIRS. With access to critical care infrastructure, advanced diagnostics, and trained personnel, hospitals facilitate rapid intervention. The rising incidence of severe infections and the emphasis on improved patient outcomes in emergency and ICU departments are expected to sustain this segment’s growth.
Market Segments
By Indication
- Urinary Tract Infection (UTI)
- Autoimmune Diseases
- Pneumonia
- Others
By End-user
- Hospitals
- Ambulatory Surgical Centers
- Specialty Clinics
- Others
Regional Analysis
North America Leads the SIRS Treatment Market
In 2024, North America accounted for 41.8% of the global systemic inflammatory response syndrome (SIRS) treatment market, maintaining its dominant position. This growth is largely driven by the high prevalence of sepsis and critical infections, along with continued advancements in critical care and rising healthcare spending. According to the CDC, sepsis affects at least 1.7 million Americans annually, with around 350,000 patients either dying or entering hospice care—underscoring the urgent need for effective SIRS therapies.
Hospitals across the United States are prioritizing early detection and treatment of sepsis. In 2023, 78% of hospitals had established sepsis committees, up from 73% in 2022, reflecting a system-wide commitment to improving outcomes. The region’s strong pharmaceutical and medical device sectors further support market growth. For example, AstraZeneca recorded US$54.07 billion in revenue in 2024, up by US$8.45 billion from 2023, reflecting increased demand for therapies targeting inflammatory and infectious conditions.
Asia Pacific Expected to Exhibit the Fastest Growth
The Asia Pacific region is projected to register the highest CAGR in the SIRS treatment market over the forecast period. Growth is fueled by a large population base, rising incidence of infectious diseases, and ongoing improvements in healthcare infrastructure. Enhanced access to diagnostics and treatment is expected to support early intervention for SIRS across the region.
According to the World Health Organization, sepsis impacts nearly 49 million people globally each year. In South Korea alone, sepsis-related deaths reached 6,928 in 2022, highlighting the region’s growing burden of severe infections. Government initiatives and investment in healthcare modernization are accelerating regional growth. The World Economic Forum’s 2024 report on Asia-Pacific healthcare systems emphasizes the strategic focus on building sustainable and resilient infrastructure.
Medical technology providers are expanding their presence in the region. Johnson & Johnson’s MedTech division which offers surgical and interventional solutions relevant to severe inflammatory care generated over US$30 billion in 2023, with continued portfolio expansion anticipated in Asia Pacific markets.
Emerging Trends
A multidisciplinary approach to sepsis and SIRS management has been formalized through the CDC’s Hospital Sepsis Program Core Elements. By integrating quality-improvement initiatives such as regular sepsis-committee meetings, antimicrobial stewardship alignment, and data-driven feedback hospital mortality rates have been shown to decline alongside reductions in length of stay and overall costs.
Time-sensitive supportive interventions are being emphasized. Current CDC guidance recommends initiation of broad-spectrum antibiotics as soon as SIRS with suspected infection is identified, and the administration of a fluid bolus of 20–30 mL/kg crystalloids within the first three hours to maintain organ perfusion and prevent progression to multiple organ dysfunction syndrome (MODS).
Extracorporeal blood purification techniques most notably cytokine hemoadsorption are under active investigation. FDA Emergency Use Authorization has been granted for devices such as CytoSorb, which in early studies has been shown to reduce circulating cytokine levels (e.g., IL-6) by up to 50% in hyperinflammatory states; these devices are now being studied in more than 50 countries.
Biomarker-guided immunomodulation is gaining traction. Measurement of inflammatory mediators (e.g., IL-6, C-reactive protein, ferritin) is being used to select patients for adjunctive therapies, with trials enrolling patients whose IL-6 levels exceed 500 pg/mL in order to optimize timing and efficacy of hemoadsorption.
Use Cases
- Early Goal-Directed Supportive Care: In a cohort of patients with severe SIRS progressing toward sepsis, a 28-day in-hospital mortality rate ranging from 10% to 40% has been reported. When broad-spectrum antibiotics are administered within the first hour of meeting SIRS criteria and fluid resuscitation at 20–30 mL/kg is completed within three hours, progression to organ failure and mortality have been shown to decline by approximately 20% in retrospective analyses.
- Cytokine Hemoadsorption in Septic Shock: In a case series of nine critically ill patients with COVID-19–associated hyperinflammation, cytokine hemoadsorption treatment reduced median plasma IL-6 levels from ~2,000 pg/mL to ~1,000 pg/mL (a 50% decrease) and improved the PaO2/FiO2 ratio from 103 to 150, facilitating better oxygenation without significant device-related complications.
- Multisystem Inflammatory Syndrome in Children (MIS-C): Following SARS-CoV-2 infection, multisystem inflammatory syndrome in children presents as a SIRS-like state with an incidence of 0.11 cases per million person-months in 2023 an 80% decline from the previous year. Treatment protocols involving intravenous immunoglobulin and corticosteroids have been associated with rapid resolution of cardiovascular inflammation, with the majority of patients regaining normal cardiac function within four weeks.
Conclusion
The global SIRS treatment market is poised for stable growth, driven by rising sepsis cases, post-surgical complications, and trauma-induced inflammation. Advancements in diagnostics, critical care infrastructure, and the adoption of immunomodulatory and extracorporeal therapies are enhancing patient outcomes.
Hospitals remain the primary end-users due to high ICU admission rates and access to advanced treatment protocols. North America leads the market, while Asia Pacific is projected to grow rapidly due to expanding healthcare access. Continued investment in precision medicine, AI-driven support tools, and biomarker-guided therapies is expected to further strengthen global efforts in managing systemic inflammatory conditions.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



