Table of Contents
Introduction
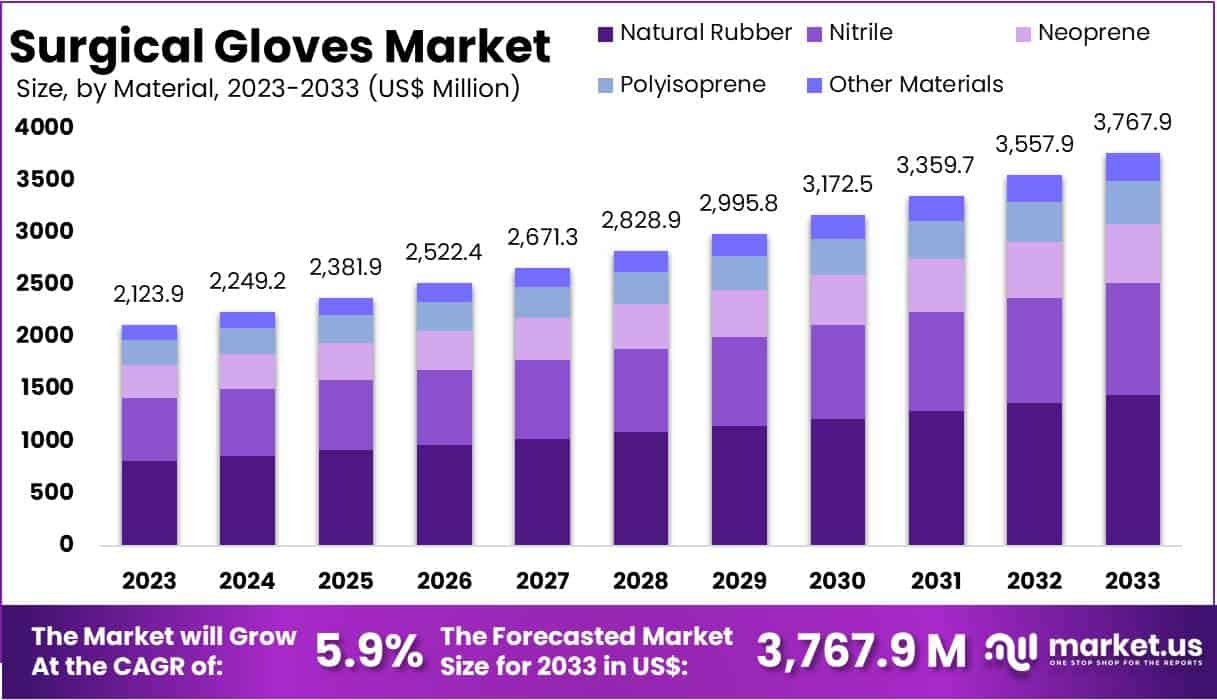
The Global Surgical Gloves Market size is expected to be worth around USD 3767.9 Million by 2032, from USD 2123.9 Million in 2023, growing at a CAGR of 5.9% during the forecast period from 2023 to 2033. In 2023, North America held over 37.8% market share, reaching a revenue total of US$ 802.8 Million.
The market’s growth is driven by several factors, including technological advancements, regulatory requirements, and evolving market demands, particularly in response to the COVID-19 pandemic.
Technological innovations are playing a pivotal role, with a growing preference for biodegradable gloves. These gloves decompose much faster than traditional synthetic options, addressing environmental concerns associated with glove disposal and promoting sustainability in healthcare. This trend reflects a broader movement towards eco-friendly medical supplies.
Regulatory bodies are implementing stricter standards to reduce surgical site infections, increasing the demand for high-quality surgical gloves that meet rigorous sterility and safety criteria. These regulations are fostering market growth by encouraging advanced manufacturing practices.
The COVID-19 pandemic has significantly boosted the demand for surgical gloves, prompting manufacturers to expand production and innovate product designs. Companies have been exploring new materials to maintain a stable supply while meeting the surge in global demand.
Material innovations are further shaping the market, driven by concerns over latex allergies among healthcare workers and patients. Manufacturers are increasingly using synthetic materials such as neoprene and polyisoprene, which mimic the properties of latex without triggering allergic reactions, thus improving user safety and comfort.
Recent developments in the market include the U.S. imposing a 50% import tariff on Chinese-made gloves starting in January 2025. This policy is expected to benefit Hartalega Holdings Bhd, potentially increasing its U.S. market share from 40%-50% to 60%-70%. Additionally, in April 2024, Ansell acquired Kimberly-Clark’s Personal Protective Equipment business for USD 640 million, a move aimed at expanding its product portfolio in safety apparel.
Key Takeaways
- The market is expected to expand from $2123.9 million in 2023 to $3767.9 million by 2032, with a 5.9% CAGR.
- Natural rubber gloves led the market in 2023, holding a 38.5% share, praised for their superior elasticity and comfort.
- Sterile gloves made up 68.5% of the market in 2023, crucial for infection prevention during surgeries.
- Powder-free gloves dominated the market in 2023 with an 88.5% share, chosen for their lower allergy and respiratory risks.
- In 2023, offline sales channels predominated, capturing 64.7% of the market, due to their immediate product availability and quality verification.
- Hospitals and clinics were the largest consumers of the product, making up 64.9% of the market in 2023, necessitated by high volumes of surgical procedures.
- The preference for disposable gloves, particularly nitrile, is growing as they help minimize contamination risks.
- North America was the market leader in 2023, holding a 37.8% share, bolstered by advanced technologies and a high number of surgical procedures.
- Forecasted to rise at a 6.5% CAGR, the Asia Pacific region is growing rapidly due to an aging population and increased healthcare investments.
- Leading manufacturers like Hartalega Holdings, Sempermed, and Ansell Ltd. are making significant investments in product innovation and manufacturing capabilities.
Surgical Gloves Statistics
- Wearing gloves for over 60 minutes significantly increases the risk of perforation.
- The Association of PeriOperative Registered Nurses (AORN) recommends changing gloves every 60 to 150 minutes to minimize failure rates.
- Surgical gloves must meet an Acceptable Quality Limit (AQL) of 0.65%, ensuring a low probability of defects such as holes.
- The EN 455 Part 1 standard requires gloves to undergo water testing to evaluate their effectiveness as barriers against microorganisms.
- EN 455 Part 2 outlines the necessary dimensions and physical strength of gloves, which vary based on material and classification.
Production and Supply Concerns
- Malaysia is the largest producer of nitrile gloves, supplying approximately 60% of the global demand.
- In 2020, Malaysia accounted for 75% of U.S. medical glove imports, excluding hard rubber gloves.
- Forced labor issues during the COVID-19 pandemic led to U.S. Customs and Border Protection restrictions on two subsidiaries of Top Glove in July 2020.
- Nitrile glove prices in Malaysia increased by 18% between July and August 2020.
- A COVID-19 outbreak at Top Glove facilities in November 2020 caused 28 out of 41 factories to temporarily close, exacerbating glove shortages in the U.S.
- Between November and December 2020, nitrile glove prices rose by 20% due to increased demand and supply chain disruptions.
Demand and Domestic Production
- The U.S. required approximately 660 million gloves for COVID-19 vaccination efforts.
- Domestic production remains insufficient, with companies like Rhino Health and Showa Group working to expand capacity.
- By 2021, U.S. manufacturers produced 6.3 billion gloves annually, but monthly imports still exceeded 5 billion gloves.
- China dominated the U.S. vinyl medical glove market by 2020, accounting for 98% of imports, and increased its share of nitrile glove imports to 19% by February 2021.
- The FDA classifies medical gloves as Class I reserved medical devices, requiring a 510(k) premarket notification to ensure compliance with leak resistance and biocompatibility standards.
- In response to health risks, the FDA banned powdered medical gloves on December 19, 2016.
Supply Chain Optimization and Cost Savings
- Health systems and academic institutions have achieved cost savings and supply chain efficiency through SKU reduction initiatives.
- Over 50 operating rooms transitioned to latex-free environments, with similar efforts in over 70 operating rooms at a regional health system.
- A nonprofit health system reduced SKUs by 60%, transitioning more than 500 operating rooms to latex-free surgical gloves.
- Customer feedback revealed that 79% of respondents reported cost savings after switching to Cardinal Health’s latex-free surgical gloves.
Emerging Trends
- Shift to Synthetic Materials: The surgical glove market is increasingly transitioning from latex to synthetic materials such as nitrile and neoprene. This shift is driven by growing awareness of latex allergies among healthcare workers and patients. Synthetic gloves not only reduce allergic reactions but also provide better puncture resistance, enhancing safety during medical procedures. Manufacturers are focusing on expanding their synthetic glove portfolios to meet the rising demand for safer, allergy-free options.
- Focus on Infection Control: Enhanced infection prevention measures are driving the adoption of advanced surgical gloves, including powder-free options that significantly lower contamination risks. These gloves align with stricter infection control protocols aimed at protecting healthcare workers and patients. Improved protective features ensure greater safety during surgical and clinical procedures, reflecting the industry’s commitment to reducing infection rates.
- Eco-Friendly Innovations: Environmental concerns are propelling the development of biodegradable and eco-friendly surgical gloves. Manufacturers are innovating to produce gloves that meet medical safety standards while addressing sustainability issues. This trend underscores the healthcare sector’s growing emphasis on environmental responsibility, as more facilities adopt sustainable practices without compromising clinical effectiveness.
- Technological Advancements in Design: Technological improvements are enhancing the tactile sensitivity, durability, and comfort of surgical gloves. These innovations aim to provide a “second-skin” feel, enabling healthcare professionals to perform intricate surgical tasks with precision. The focus is on creating gloves that combine flexibility and superior protection to meet the rigorous demands of modern surgical procedures.
- Regulatory and Educational Progress: The market is influenced by stricter regulatory standards and increased educational initiatives promoting proper glove usage. Improved regulations ensure compliance with high safety and quality benchmarks, while training programs raise awareness among healthcare providers. These efforts enhance the effective use of surgical gloves, contributing to better safety outcomes in medical and surgical environments.
Use Cases
- Healthcare Facilities: Surgical gloves are indispensable in hospitals and clinics for maintaining safety and hygiene during surgeries and examinations. They act as a barrier against pathogens, preventing infections among healthcare professionals and patients. Their use is critical in ensuring sterile conditions in medical settings.
- Diagnostic and Research Laboratories: In laboratories, surgical gloves are vital for preventing cross-contamination while handling samples and chemicals. They help maintain the integrity of experiments and ensure the safety of lab personnel. Their use is essential in achieving accurate test results and protecting scientific data.
- Emergency Responders: Emergency medical technicians and first responders rely on surgical gloves to protect themselves and patients from infections during medical emergencies. Gloves provide a protective barrier against pathogens, enabling responders to perform their duties safely and effectively.
- Dental Practices: In dental care, surgical gloves are crucial for preventing infection transmission during procedures. They protect both patients and dental professionals by maintaining a sterile environment. From examinations to surgical treatments, gloves are a standard safeguard in dental practices.
- Veterinary Medicine: Surgical gloves are essential in veterinary care to prevent disease transmission between animals and staff. They ensure sanitary conditions during treatments and surgeries, safeguarding both animal patients and veterinary professionals. Gloves are vital in maintaining health standards across diverse veterinary settings.
Conclusion
The surgical glove market is experiencing significant growth driven by advancements in technology, regulatory requirements, and evolving healthcare demands. Factors such as the shift to synthetic materials, eco-friendly innovations, and enhanced infection control measures underscore the industry’s commitment to safety and sustainability. The COVID-19 pandemic amplified the demand for gloves, spurring innovations in materials and production.
Additionally, emerging markets like Asia Pacific show strong growth potential due to increasing healthcare investments. With regulatory progress and technological advancements, the market is well-positioned to address rising safety, environmental, and efficiency needs, cementing its critical role in global healthcare and medical practices.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



