Table of Contents
Introduction
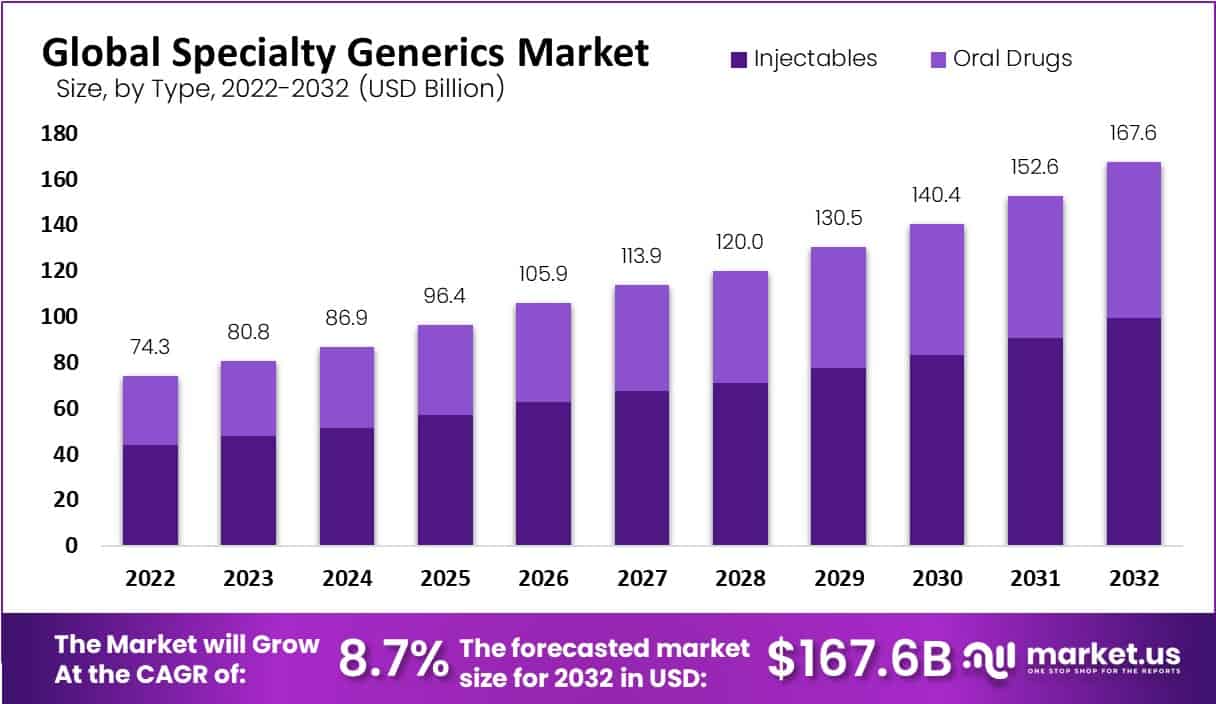
The Specialty Generics Market is projected to grow from USD 74.3 billion in 2022 to USD 167.6 billion by 2032, with a CAGR of 8.7%. This market is expanding due to several key factors, including regulatory support, technological advancements, and increased focus on accessibility and affordability.
Regulatory agencies like the FDA are enhancing the development of complex generics. By finalizing guidelines that clarify approval pathways, they reduce uncertainties and expedite the manufacturing process. This regulatory support is crucial for companies navigating the complexities of producing generics with sophisticated mechanisms or delivery methods.
Technological innovations are pivotal in overcoming production challenges associated with these complex generics. Advances in drug development tools and manufacturing technologies not only make production feasible but also facilitate market entry, thereby expanding the range of generic medications available.
The emphasis on making essential medicines accessible and affordable, particularly in low- and middle-income countries, is driving the generics market. Strategies are being developed to improve drug registration and availability, which are essential for addressing global health disparities and ensuring that patients can afford the medications they need.
Finally, collaborations between regulatory bodies, academia, and the pharmaceutical industry are fostering advancements in complex generic drugs. These partnerships help overcome challenges in drug formulation and bioequivalence studies, leading to innovations that enhance the quality and availability of generic medications. Moreover, advocacy by various organizations promotes policies that support generic competition and streamline approval processes, ensuring continuous drug availability amid market and supply chain challenges.
Key Takeaways
- The Specialty Generics Market is projected to reach USD 167.6 billion by 2032, growing at a steady CAGR of 8.7% starting from 2023.
- Market growth is significantly influenced by the increasing prevalence of chronic diseases, boosting demand for specialty generics.
- The injectables segment is leading the market because they are absorbed quickly, resulting in the highest revenue share.
- Growth in the inflammatory conditions segment is notable, driven by a rise in cases of such diseases.
- Specialty pharmacies are pivotal in the market for their low distribution costs and enhanced accessibility.
- There is promising market potential in emerging regions of Asia and Latin America, primarily due to existing unmet healthcare needs.
- Drug manufacturers are focusing on innovation, developing new drug administration techniques to stand out in the market.
Emerging Trends and Use Cases
- Development of Complex Generics: Complex generics include advanced formulations like peptides, liposomes, or drug-device combinations. Their development is challenging due to intricate components and rigorous approval requirements. Ensuring bioequivalence to brand-name drugs demands specialized techniques and adherence to strict regulatory standards. These challenges underscore the need for advanced technology and guidance in developing high-quality alternatives. Complex generics play a pivotal role in treating chronic and rare conditions, improving access to essential medications for patients globally.
- Support from the FDA: The FDA actively supports generic drug manufacturers by offering product-specific guidances. These documents provide insights into developing therapeutically equivalent generics for specific reference drugs. By clarifying expectations and regulatory pathways, the FDA helps streamline the approval process. This support ensures that complex generics meet high safety and efficacy standards, enabling quicker market access. Patients benefit from timely access to affordable alternatives to brand-name medications.
- Economic Impact and Accessibility: Specialty generics significantly reduce healthcare costs, saving billions for the U.S. healthcare system annually. They constitute a substantial share of prescriptions filled, enhancing affordability and accessibility. For patients with chronic conditions, these cost-effective alternatives ensure consistent access to life-saving medications. By reducing financial strain, specialty generics improve healthcare equity, allowing broader patient populations to benefit from essential treatments.
- Regulatory Advancements and Challenges: The U.S. Pharmacopeia (USP) collaborates with stakeholders to address challenges in developing complex generics. Efforts focus on refining scientific and regulatory frameworks, reducing market-entry barriers, and promoting innovation. These initiatives aim to expand the availability of specialty generics while ensuring affordability. Regulatory advancements foster competition, improve quality, and enhance patient trust in these alternatives. This collaborative approach benefits the entire healthcare system, supporting long-term sustainability.
Conclusion
The Specialty Generics Market is poised for remarkable growth, driven by advancements in technology, regulatory support, and the increasing focus on accessibility and affordability. These generics play a vital role in reducing healthcare costs and improving access to essential medicines, particularly for chronic and rare conditions. Innovations in complex formulations, supported by guidance from regulatory agencies like the FDA, are fostering faster approvals and market entry. Collaboration among industry stakeholders is also enhancing drug quality and availability. With growing demand across emerging regions and evolving healthcare needs, specialty generics are set to address critical gaps in global healthcare, ensuring equitable access to affordable and life-saving treatments for diverse patient populations.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



