Table of Contents
Overview
New York, NY – Aug 04, 2025: The Global Small Molecule CDMO Market is projected to reach US$ 136.7 billion by 2034, up from US$ 68.2 billion in 2024. This growth, at a CAGR of 7.2% from 2025 to 2034, is driven by a mix of market and technical dynamics. Key factors include rising demand for generic drugs, complex drug formulations, and the shift towards outsourcing. These trends are reshaping pharmaceutical manufacturing, creating new opportunities for CDMOs. The industry is evolving fast, with specialized partners playing a growing role in drug development and production.
Generic drug demand is a major growth driver. The U.S. FDA reports that generics now account for 90% of all filled prescriptions. As brand-name drug patents expire, pharma companies must introduce cost-effective alternatives. CDMOs offer scalable manufacturing solutions to meet this need. Their flexible infrastructure supports faster production cycles. As pricing pressures increase, manufacturers are turning to CDMOs to stay competitive. This trend is likely to intensify as more blockbuster drugs lose exclusivity over the next decade.
Modern drug development has become highly complex. Many small molecule therapies target rare diseases or involve potent active ingredients. These formulations require specialized facilities and technical know-how. In-house teams often lack the necessary capabilities, making outsourcing essential. CDMOs are equipped with advanced technologies to handle high-potency APIs and other niche compounds. This not only reduces development risks but also speeds up production timelines. As a result, biopharma firms are relying more on external partners to manage technical challenges efficiently.
Strategic outsourcing is another key factor in CDMO market growth. Pharma companies are focusing more on R&D and commercialization, while outsourcing manufacturing to reduce costs. This approach cuts capital expenditure and shortens time-to-market. A Deloitte study found that outsourcing can reduce development timelines by 20%. In 2022 alone, outsourcing spend topped US$ 700 billion. This figure is expected to rise to US$ 731 billion by 2025. The continued shift towards leaner operating models makes CDMOs a critical part of the pharmaceutical value chain.
Supportive regulatory frameworks also help drive market expansion. Agencies like the FDA and EMA have streamlined drug approval processes. Programs like the FDA’s GDUFA have accelerated generic drug reviews. These efforts improve access while maintaining safety standards. Faster approvals mean quicker product launches and higher demand for CDMO services. Regulatory clarity reduces uncertainty and supports long-term investment in CDMO capabilities. Together, these factors create a favorable environment for sustained growth in the global Small Molecule CDMO Market.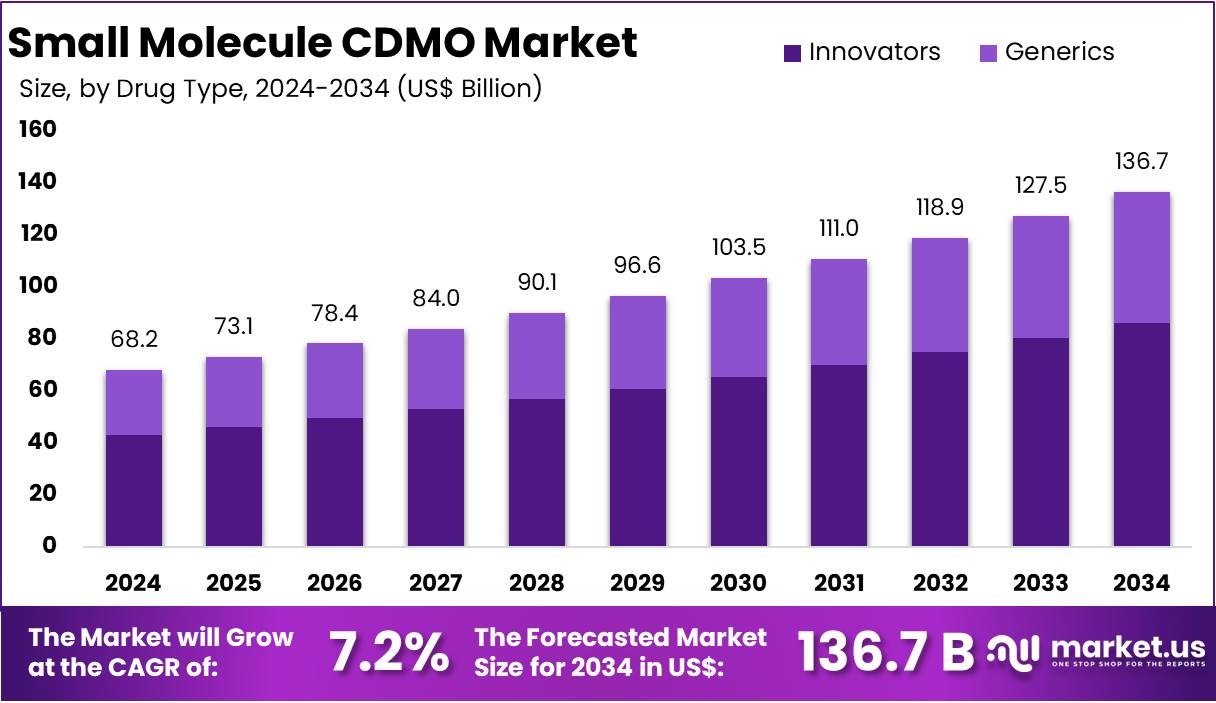
Key Takeaways
- According to analysts, the global Small Molecule CDMO market is expected to hit US$ 136.7 billion by 2034, growing at 7.2% annually.
- In 2024, Active Pharmaceutical Ingredients (API) led product categories, contributing over 63.2% of the market share, driven by rising generic drug demand.
- Industry experts note that Innovator drugs dominated the drug type category in 2024, making up more than 56.8% of total market revenue.
- Oncology was the top application area in 2024, accounting for over 32.5% of the market, largely due to increasing targeted therapy development.
- North America retained its leadership position in 2024, holding 42.7% market share, with regional revenues surpassing US$ 29.1 billion that year.
Regional Analysis
North America led the global Small Molecule CDMO market with a commanding 42.7% share, valued at US$ 29.1 billion. This stronghold is largely driven by the region’s advanced pharmaceutical manufacturing infrastructure and the presence of prominent CDMOs and biotech innovators. The increasing trend of outsourcing among pharmaceutical companies has further fueled regional expansion. The United States, in particular, plays a pivotal role due to its favorable regulatory framework under the U.S. FDA, which accelerates drug development and approval processes.
The region’s dominance is reinforced by high R&D spending and a growing focus on clinical trials by major pharmaceutical players. North American CDMOs are increasingly offering integrated services tailored to the rising demand for personalized and targeted therapies. Capabilities in handling complex small molecule APIs and oral solid dosage forms are key differentiators. Combined with the availability of advanced manufacturing technologies and a skilled workforce, these factors are expected to keep North America at the forefront of the Small Molecule CDMO market throughout the forecast period.
Segmentation Analysis
Active Pharmaceutical Ingredients (APIs) dominated the product segment of the Small Molecule CDMO market, capturing over 63.2% of the total share. This growth stems from rising outsourcing needs as pharmaceutical firms reduce in-house production to cut costs and focus on R&D. CDMOs offer both large-scale API synthesis and compliance with strict regulatory standards, making them ideal partners. Regulatory agencies like the FDA and EMA continue to tighten quality controls, prompting more companies to collaborate with CDMOs to ensure safe, consistent API manufacturing.
Within the drug type segment, Innovators led the market in 2024, contributing more than 56.8% to overall revenue. This leadership is due to growing demand for novel, complex small molecule drugs. CDMOs support these firms with integrated services, from formulation to commercial manufacturing. Over 50 new chemical entities were approved globally in 2023, requiring advanced CDMO capabilities. While generics held a smaller share, they remain essential especially in emerging markets despite facing pricing pressures and tighter profit margins. CDMOs remain key partners for generic drugmakers seeking cost-effective and compliant production support.
By application, Oncology was the top-performing segment in 2024, securing more than 32.5% market share. This is largely due to the increasing cancer burden and the complexity of oncology drug development, which requires specialized CDMO services. Cardiovascular drugs followed closely, driven by the global prevalence of heart disease. CDMOs help accelerate development of both novel and generic cardiovascular therapies. Central Nervous System (CNS) drugs also held a notable share, with demand rising for treatments targeting disorders like
Key Players Analysis
The Small Molecule CDMO market is highly competitive, led by global powerhouses and supported by specialized regional firms. Key players are expanding capacities and investing in advanced technologies to meet rising industry demands. Lonza stands out with its strong API and cytotoxic development capabilities, supported by state-of-the-art facilities in the U.S. and Switzerland. Catalent, Inc. offers end-to-end services and has significantly boosted its global footprint through investments in solid dose technologies and strategic acquisitions, particularly across Europe and the Asia-Pacific region.
Thermo Fisher Scientific maintains a robust market position through its integrated CDMO services, including API synthesis, formulation, and sterile fill-finish. The company has prioritized oncology and CNS therapies through targeted investments. Cambrex Corporation focuses on small molecule APIs and U.S.-based manufacturing, offering early-stage development solutions and continuous flow chemistry. Meanwhile, Bellen Chemistry, based in China, is gaining momentum with cost-effective preclinical and IND-enabling services. Other notable players include WuXi STA, Eurofins CDMO, Siegfried Holding AG, Alcami Corporation, and Olon Group—each offering regional strengths, digital manufacturing solutions, and full-spectrum support across all phases of small molecule drug development.
Emerging Trends
- More Pharma Companies Are Outsourcing Production: Pharmaceutical companies are outsourcing more of their small molecule drug manufacturing. This helps reduce operational costs and eliminates the need for expensive manufacturing setups. By working with CDMOs, pharma companies can focus more on research and drug discovery. CDMOs offer the experience, equipment, and infrastructure required to meet global production standards. This approach also improves flexibility and speeds up time-to-market. As competition increases in drug development, outsourcing has become a strategic way to stay ahead. CDMOs allow companies to manage risks better while meeting growing market demands. This trend is expected to grow as pipelines expand and efficiency becomes more critical.
- Growing Focus on Complex Molecules: CDMOs are now handling more complex small molecule drugs. These include high-potency compounds and therapies for rare or difficult-to-treat diseases. Producing such drugs requires strict safety protocols and advanced technologies. Many pharmaceutical firms lack the facilities or expertise to manage these compounds in-house. That’s where CDMOs come in. They offer cleanrooms, containment systems, and specialized staff to safely develop and manufacture these complex molecules. As demand for targeted and precision medicines grows, so does the need for CDMOs with niche capabilities. This trend reflects the shift from high-volume drugs to more specialized treatments with unique manufacturing needs.
- End-to-End Service Demand Is Rising: Clients are now looking for CDMOs that can handle the entire drug development process. This includes everything from initial formulation to commercial production. Working with a single partner makes the process faster and smoother. It reduces the risk of delays and improves communication across different stages. CDMOs offering end-to-end services help companies save time and money. These services also allow better control of quality and regulatory compliance. As more drugs move from lab to market, having one provider manage the journey is more efficient. This trend shows how important integrated service offerings have become in the CDMO space.
- Green Chemistry and Sustainable Manufacturing: Sustainability is becoming a key priority in pharmaceutical manufacturing. Many CDMOs are adopting green chemistry techniques to reduce environmental impact. This includes using safer solvents, reducing chemical waste, and improving energy efficiency. Regulatory bodies are encouraging eco-friendly practices, and pharma clients are demanding greener supply chains. CDMOs that follow sustainable practices also reduce operational risks and long-term costs. These efforts not only help the environment but also enhance brand image. As the push for sustainability grows worldwide, CDMOs that prioritize eco-conscious production are gaining a competitive edge in the market.
- Digitalization and Smart Manufacturing: CDMOs are adopting digital tools to improve their processes. This includes automation, real-time monitoring, and smart data systems. Digitalization helps reduce errors and improve production efficiency. With better data tracking, CDMOs can ensure consistent quality and faster decision-making. Smart systems also improve equipment maintenance and resource use. Clients benefit from greater transparency and faster turnaround times. As the pharmaceutical industry becomes more tech-driven, CDMOs that invest in digital transformation are staying ahead. This trend shows how digital tools are reshaping manufacturing and making it more reliable, efficient, and scalable.
Use Cases
- Launch of a New Anti-Cancer Drug: A biotech company partnered with a CDMO to launch a small molecule cancer drug. The CDMO managed every step, from API synthesis to final capsule production. This full-service approach helped the company reduce its launch timeline by eight months. It also lowered operational costs by around 25%. By outsourcing to a specialized team, the biotech firm avoided costly infrastructure upgrades. This allowed them to focus on regulatory approvals and marketing strategies. Faster production meant they could reach patients sooner and stay ahead of competitors. The partnership proved crucial for both speed and efficiency in bringing the drug to market.
- Switch from In-House to Outsourced API Manufacturing: A pharmaceutical company decided to outsource API manufacturing for five of its generic drugs. Working with a CDMO helped them save over US$ 12 million each year. These savings came from reduced labor costs and eliminating the need for facility maintenance. Quality remained consistent due to the CDMO’s robust compliance systems. This shift also freed up internal resources, which were redirected to research and development. By outsourcing, the company could scale production more easily without investing in new infrastructure. This strategy improved their cost structure and maintained product reliability across large volumes.
- Clinical Trial Material Supply: During a Phase II trial, a startup needed 15,000 units of a test oral tablet. The CDMO handled everything manufacturing, packaging, and delivery in just 10 weeks. This quick turnaround kept the clinical trial on schedule. The CDMO also ensured all regulatory requirements were met during production. Their fast response helped the startup avoid costly delays. In-house manufacturing would have taken much longer due to setup time and limited resources. The partnership with the CDMO gave the startup the flexibility and speed needed for a time-sensitive trial. It also improved the chances of moving into the next trial phase.
- Tech Transfer of a High Volume Drug: A large pharmaceutical company moved production of a high-volume diabetes drug to a CDMO in Asia. The CDMO now produces over 10 million tablets every month. This transfer improved supply chain flexibility and reduced costs by 18%. The company no longer had to manage the day-to-day operations of large-scale production. Instead, it focused on marketing and global distribution. The CDMO ensured quality standards and met global regulatory expectations. The partnership made it easier to scale production as demand increased. This case highlights how CDMOs support large product volumes with cost-effective, reliable manufacturing solutions.
FAQs About Small Molecule CDMO
1. What is a Small Molecule CDMO?
Ans:- A Small Molecule CDMO (Contract Development and Manufacturing Organization) is a company that helps pharmaceutical firms develop and produce small molecule drugs. These drugs are chemically synthesized and typically have low molecular weight. CDMOs provide services like formulation, API production, and final drug manufacturing.
2. How do Small Molecule CDMOs support pharmaceutical companies?
Ans:- They offer end-to-end solutions—from early-stage development to commercial manufacturing. CDMOs help reduce costs, speed up time-to-market, and manage regulatory compliance.
3. What types of services do Small Molecule CDMOs offer?
Ans:- Services include API synthesis, formulation development, stability testing, analytical services, clinical trial material production, and commercial-scale manufacturing. Some also offer packaging and regulatory support.
4. Why do companies outsource to CDMOs instead of producing in-house?
Ans:- Outsourcing saves money, reduces operational burden, and allows companies to focus on drug discovery and marketing. CDMOs also offer specialized expertise and infrastructure that many firms may not have internally.
5. What kinds of drugs are considered small molecules?
Ans:- Small molecule drugs include most traditional medications like tablets, capsules, and oral liquids. Examples are painkillers, antibiotics, and cancer drugs. They are different from large molecule drugs like biologics or vaccines.
6. What is driving growth in the Small Molecule CDMO market?
Ans:- Market growth is driven by increasing demand for outsourcing, complex drug development needs, rising generic drug production, and regulatory compliance requirements.
7. Which regions dominate the Small Molecule CDMO market?
Ans:- North America currently holds the largest share, followed by Europe and Asia-Pacific. Growth in Asia is rising due to cost advantages and expanding manufacturing capacity.
8. What sectors rely most on Small Molecule CDMOs?
Ans:- Oncology, cardiovascular diseases, central nervous system (CNS) disorders, and infectious diseases are the top therapeutic areas using CDMO services.
9. Who are the major players in the Small Molecule CDMO market?
Ans:- Key companies include Lonza, Catalent, Thermo Fisher Scientific, Cambrex, WuXi STA, Siegfried Holding AG, and Eurofins CDMO, among others.
10. What trends are shaping the future of the CDMO market?
Ans:- Emerging trends include digitalization, green chemistry practices, personalized medicine, complex API manufacturing, and increasing demand for end-to-end solutions.
Conclusion
In conclusion, the global Small Molecule CDMO market is poised for significant growth, expected to double in value from US$ 68.2 billion in 2024 to US$ 136.7 billion by 2034, driven by a 7.2% CAGR. This expansion is fueled by rising demand for generics, complex drug formulations, and a strategic shift toward outsourcing.
CDMOs are playing a critical role in modern pharmaceutical development by offering flexible, end-to-end services, advanced manufacturing technologies, and regulatory expertise. As trends like digitalization, green chemistry, and personalized medicine reshape the industry, CDMOs are becoming indispensable partners helping biopharma firms improve efficiency, reduce costs, and bring innovative therapies to market faster.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



