Table of Contents
Overview
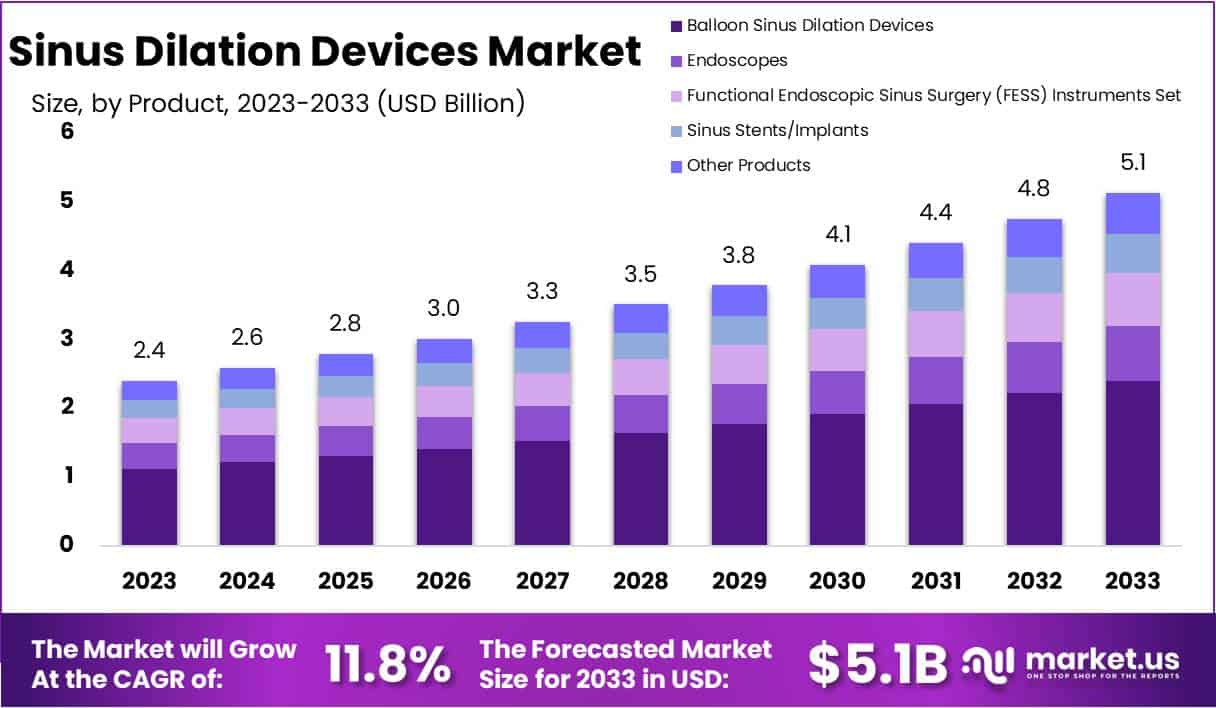
The global sinus dilation devices market is projected to reach USD 5.1 billion by 2033, increasing significantly from USD 2.4 billion in 2023. This growth reflects a CAGR of 11.8% during 2024–2033. The expansion is primarily driven by the persistent burden of chronic sinusitis, affecting millions worldwide. In the United States alone, an estimated 28.9 million adults are diagnosed with sinusitis, representing around 11.6% of the adult population. Such high prevalence sustains strong demand for minimally invasive treatment options.
Ageing populations are a major factor shaping long-term demand. The World Health Organization projects that by 2030, one in six individuals will be over 60 years old, and the population aged 80 years and above will triple by 2050. Older adults are more prone to chronic upper-airway diseases and tend to seek durable, low-risk treatment solutions. The adoption of balloon-based dilation techniques is supported by this demographic trend, ensuring procedure growth across developed and emerging regions alike.
Environmental exposures also intensify market expansion. WHO estimates that ambient air pollution caused 4.2 million premature deaths in 2019, with combined household and outdoor pollution linked to 6.7–7 million annual deaths. Poor air quality worsens rhinitis and sinusitis symptoms, creating a higher number of patients referred for definitive procedures. While air quality policies are unevenly enforced worldwide, persistent respiratory morbidity is expected to sustain treatment volumes and device utilization.
Allergy prevalence further contributes to market momentum. Studies report that 10–30% of adults globally suffer from allergic rhinitis, which is closely linked to chronic sinusitis. Increased allergy loads exacerbate mucosal inflammation, obstruction, and disease progression. This enlarges the pool of patients who qualify for sinus dilation procedures. As allergy incidence continues to climb, the related burden strengthens the demand for effective and minimally invasive interventions.
System Dynamics, Policies, and Innovation
Healthcare system dynamics are increasingly favorable for sinus dilation adoption. Long waiting times and backlogs, particularly in public health systems such as the UK’s NHS, highlight the need for efficient, office-based procedures. Balloon dilation offers shorter recovery, reduced dependence on inpatient resources, and faster turnaround for patients. Such attributes align with outpatient care trends and reinforce market expansion in resource-constrained systems.
Policy and guideline developments also strengthen adoption. In England, NICE has acknowledged balloon dilation as safe and effective when used under governance standards. This recognition within the public health system lowers barriers for adoption and supports device commissioning. Similarly, in the United States, the FDA has granted multiple clearances for sinus balloon systems under the 510(k) pathway since 2005. Recent approvals in 2023 confirm a continuous cycle of innovation and competition, expanding clinical options.
Coding and reimbursement frameworks provide another critical enabler. Established CPT codes ensure smooth integration into outpatient billing systems, while historical CMS documentation has recognized balloon sinuplasty within Medicare policies. These reimbursement structures lower financial risks for providers and encourage procedural uptake. Stable coding systems remain an essential factor driving predictable growth.
Global antimicrobial resistance (AMR) strategies indirectly boost demand for sinus dilation. Recurrent sinus infections often require repeated antibiotic courses, which contribute to resistance development. WHO identifies AMR as a major global threat, responsible for 1.27 million direct deaths in 2019. Sinus ostial dilation offers a structural solution that reduces infection recurrence and antibiotic dependency. Alignment with stewardship goals reinforces procedure value and accelerates adoption across healthcare systems.
Finally, improvements in healthcare datasets enhance market confidence. Agencies such as AHRQ and the CDC provide visibility into sinus disease visit volumes and treatment trends. Reliable data improves payer planning, hospital budgeting, and clinical decision-making. Together, high disease prevalence, supportive guidelines, reimbursement structures, and strong innovation cycles create a stable and optimistic growth outlook for the sinus dilation devices market.
Key Takeaways
- The market is projected to reach USD 5.1 billion by 2033, expanding at a compound annual growth rate (CAGR) of 11.8%.
- Balloon Sinus Dilation Devices dominated in 2023 with a 46.8% market share, highlighting their strong adoption across sinus treatment procedures worldwide.
- Standalone procedures held a 67.4% share in 2023, showing patient and physician preference for streamlined, less complex sinus treatment approaches.
- Adults accounted for 67.9% of the market in 2023, indicating sinus-related conditions are more prevalent in this demographic segment.
- Hospitals captured 53.1% of the market in 2023, reaffirming their role as primary providers of comprehensive sinus care and advanced medical procedures.
- Market growth is driven by increasing global chronic sinusitis cases, enhanced technology adoption, and rising awareness of minimally invasive sinus procedures.
- High procedural costs and strict regulatory requirements remain key challenges, restricting broader adoption and limiting access to sinus treatment procedures.
- Growth opportunities lie in expanding elderly populations and strengthening healthcare infrastructure across emerging economies, fostering market penetration and wider procedural adoption.
- Balloon sinuplasty is gaining importance as a preferred treatment, while industry collaborations focus on collective expertise in product development and innovation.
- North America led with 40.1% share in 2023, supported by high sinusitis prevalence, advanced healthcare systems, and strong procedural adoption rates.
Regional Analysis
In 2023, North America secured a leading position in the Sinus Dilation Devices Market with over 40.1% share, valued at USD 1.3 billion. The region’s dominance was supported by a high prevalence of chronic sinusitis. A growing patient base with persistent sinus conditions increased the demand for advanced treatment devices. The adoption of minimally invasive techniques became widespread due to patient preference for reduced risks and faster recovery. This demand significantly boosted the market’s expansion across the region.
Technological innovation has been a critical factor behind North America’s market leadership. The region’s healthcare systems have consistently integrated advanced sinus dilation devices. Minimally invasive procedures have been increasingly favored by both physicians and patients. With state-of-the-art medical infrastructure and skilled specialists, the transition to newer treatment options has been seamless. The continuous development of innovative devices and strong adoption rates have reinforced North America’s leadership in the global sinus dilation devices market.
Reimbursement policies in North America played a decisive role in market growth. Favorable healthcare coverage enabled easier access to advanced sinus dilation procedures. This encouraged wider adoption among patients and providers. In addition, the region has witnessed strategic collaborations between research organizations, healthcare institutions, and market players. Such initiatives focused on product development and distribution, strengthening the overall market structure. These alliances have enhanced innovation pipelines and ensured robust availability of sinus dilation solutions across the region.
Awareness campaigns and educational programs further contributed to higher diagnosis and treatment rates. Patients are now more informed about available treatment options, driving demand for sinus dilation devices. The presence of global key players in North America also intensified competition. Companies actively invested in research and development to differentiate their offerings through technology. This competition encouraged rapid advancements in device design and functionality. Supported by a strong healthcare ecosystem, North America is expected to maintain its leading role in the sinus dilation devices market.
Segmentation Analysis
In 2023, Balloon Sinus Dilation Devices held a dominant market share of 46.8%, confirming their strong preference in sinus dilation procedures. The segment’s leadership highlights the growing adoption of balloon-based devices due to their proven effectiveness in minimizing invasiveness and ensuring successful patient outcomes. Endoscopes also contributed significantly, supporting precision in navigation and visualization during sinus interventions. Functional Endoscopic Sinus Surgery (FESS) instrument sets further strengthened the market, reflecting their indispensable role in enhancing procedural efficiency and advancing minimally invasive surgical outcomes across multiple healthcare settings.
The procedure analysis showed that standalone interventions dominated the market with more than 67.4% share in 2023. This dominance reflects the preference for procedures that are simple, effective, and less complex compared to hybrid alternatives. Standalone approaches gained traction due to shorter recovery times and lower procedural risks. Hybrid procedures, although effective in selected cases, secured a smaller share of the market. The trend underscores strong confidence among healthcare providers and patients in standalone procedures, establishing them as the most widely accepted approach in sinus dilation practices worldwide.
From an application perspective, adults represented the largest consumer group, accounting for 67.9% share in 2023. The high prevalence of sinus-related disorders among adults has directly driven the adoption of sinus dilation devices within this demographic. Growing awareness and proven device effectiveness in treating adult sinus conditions further strengthened this dominance. Pediatric applications accounted for 32.1% share, emphasizing their continued importance despite smaller volume. The pediatric market remains vital, as device innovation increasingly addresses the specific needs of children, reflecting a balanced yet adult-driven growth trajectory within the overall market landscape.
In terms of end use, hospitals secured the leading share at 53.1% in 2023, supported by advanced infrastructure and comprehensive treatment facilities. Hospitals benefited from large patient inflows and trust in specialized care delivery. ENT clinics and in-office procedures also grew significantly, offering convenient and specialized services for sinus-related treatments. Ambulatory Surgical Centers (ASCs) emerged as efficient alternatives, combining outpatient convenience with quality surgical care. This diverse end-use landscape highlights hospitals’ dominance while signaling strong growth opportunities for specialized ENT and ASC facilities, indicating a shift toward more flexible and patient-centric healthcare delivery models.
Key Players Analysis
The Sinus Dilation Devices Market is witnessing consistent growth, driven by the strategic presence of global players. Companies such as B. Braun Melsungen AG, Olympus Corporation, Johnson & Johnson, and Medtronic Plc. play crucial roles in shaping the industry. Their collaborative efforts, technological advancements, and focus on patient-centered solutions contribute significantly to market expansion. The collective emphasis on innovation and improved outcomes has strengthened the overall dynamism of this segment, ensuring that both healthcare practitioners and patients benefit from evolving treatment solutions.
B. Braun Melsungen AG demonstrates strong commitment to research and development in sinus dilation devices. The company focuses on advanced technologies and patient-centric product innovation, enabling better treatment outcomes. Its solutions are designed to enhance procedural efficiency and safety, aligning with the evolving needs of healthcare providers. By prioritizing continuous innovation and quality standards, B. Braun maintains a competitive edge. This strategic emphasis contributes directly to the broader growth of the Sinus Dilation Devices Market, reinforcing the company’s influence within the global healthcare industry.
Olympus Corporation brings strong technological expertise and innovation to the Sinus Dilation Devices Market. The company is widely recognized for advancements in precision instruments and imaging technologies. Its sinus dilation solutions are designed to improve the accuracy and effectiveness of minimally invasive procedures. Olympus addresses the growing demand for efficient and reliable treatment solutions by combining innovation with clinical expertise. This commitment to quality and performance ensures its strong positioning in the global market, meeting the rising expectations of healthcare professionals and driving patient satisfaction.
Johnson & Johnson and Medtronic Plc. further reinforce the growth of the Sinus Dilation Devices Market through their global presence and diverse product portfolios. Johnson & Johnson leverages its established brand and resources to deliver effective solutions for sinus care. Medtronic Plc. offers a comprehensive range of devices that address varied patient needs in sinus interventions. Both companies emphasize innovation and accessibility, ensuring better treatment outcomes worldwide. Their combined strategic initiatives continue to drive innovation, expand market reach, and shape the long-term trajectory of the sinus dilation segment.
Leading Market Key Players
- Accurate Surgical & Scientific Instruments Corporation
- B. BRAUN MELSUNGEN AG
- Olympus Corporation
- Johnson & Johnson
- Medtronic Plc.
- Stryker Corporation
- Smith & Nephew Plc.
- Sinusys Corporation
- Sklar Surgical Instruments
- Intersect Inc.
Challenges
1) Patient Selection and Clinical Governance
Careful patient selection is critical in sinus dilation procedures. Professional guidelines strongly discourage use when no clear disease is present. Physicians are urged to conduct pre-procedure CT scans and confirm indications before treatment. This ensures safety but also adds extra steps to the workflow. These requirements reduce unnecessary use but may limit patient throughput. As a result, providers must balance clinical rigor with efficiency. Clear governance protocols are essential to maintain standards while avoiding overuse. This challenge highlights the importance of aligning patient eligibility with evidence-based practice to ensure safe and appropriate treatment outcomes.
2) Safety and Complication Vigilance
Although major complications from sinus dilation are rare, they can be very serious. Risks include orbital injury or skull base damage, both of which can have severe consequences. Adverse event reports and case series have stressed these dangers. Because of this, strong training programs and strict imaging protocols are vital. Providers are expected to monitor patients closely and maintain a high standard of procedural safety. The challenge lies in ensuring consistent vigilance while scaling adoption. Safety awareness, coupled with well-structured protocols, helps reduce complications. It also builds patient and payer confidence in the long-term reliability of this therapy.
3) Coverage Variability and Coding Complexity
Insurance coverage for sinus dilation devices is inconsistent across the United States. Medicare has not issued a National Coverage Determination specifically for balloon sinus dilation. Instead, decisions are left to local carriers or individual payers. This creates uncertainty for both providers and manufacturers. Coding and reimbursement pathways are often complex, making financial planning difficult. Some regions may offer broad coverage, while others impose restrictions. This patchwork of policies can limit patient access and slow market adoption. Addressing variability in coverage and streamlining coding practices remain critical for wider acceptance and smoother integration into healthcare systems.
4) Payer Scrutiny of “Stand-Alone” Dilation
Coverage of stand-alone sinus dilation procedures remains under close payer review. Some insurers limit reimbursement or apply evolving rules for these cases. Specialty societies have urged consistent coverage to improve access. However, payer skepticism continues, slowing adoption in certain markets. Providers face uncertainty when offering the procedure as a stand-alone option, particularly without concurrent sinus surgery. This scrutiny can affect practice economics and patient acceptance. Until payers align policies more consistently, providers must navigate complex reimbursement environments. Market growth depends on building stronger evidence to support stand-alone procedures and convincing payers of their long-term value.
5) Regulatory and Compliance Exposure
Sinus dilation devices operate under strict regulatory oversight. The FDA has issued communications and warning letters emphasizing compliance with device-use conditions. Age restrictions, indication limits, and reprocessing rules must be followed closely. Any deviation, such as off-label use, increases enforcement risk. Manufacturers and providers face exposure if compliance protocols are weak. This makes robust training, documentation, and adherence to guidelines essential. The challenge is not only meeting regulatory requirements but also maintaining trust with regulators. Failure to comply can damage reputations and delay market progress. Clear compliance systems reduce exposure and support sustainable market adoption.
6) Competitive Clinical Pathways
The sinus dilation device market faces competition from newer drug-based treatments. For example, FDA-approved fluticasone devices such as Xhance are used in chronic rhinosinusitis patients. These therapies may delay or reduce the need for surgery in some groups. Patients may prefer less invasive options, especially when drug-based treatments are effective. This creates pressure on device positioning within treatment pathways. To remain competitive, manufacturers must highlight procedural advantages. Emphasizing long-term outcomes, reduced recurrence, and suitability for refractory cases will be key. Competitive pressures require clear differentiation and careful alignment with evolving clinical practice patterns.
7) Evidence Heterogeneity and Legacy Guidance
The evidence base for sinus dilation is still uneven across settings and sinus types. Older guidance documents, such as early NICE Interventional Procedure Guidance (IPG), remain cautious. This has contributed to slower adoption in some regions. Newer studies show promising results, but heterogeneity in data quality limits consensus. This lack of harmonization creates uncertainty for clinicians, payers, and policymakers. Stronger randomized controlled trials and long-term outcome data are needed. Until then, market growth will remain constrained by legacy conservatism. Aligning global guidelines with emerging evidence is necessary to establish clearer confidence in sinus dilation devices.
Opportunities
1. Large, Stable Addressable Population
Chronic sinus disease affects millions worldwide and remains highly prevalent. Patients often experience recurring symptoms that do not fully resolve with standard medical therapies. This creates a consistent demand for treatment options. Sinus dilation procedures can address these unmet needs, offering relief where drugs may not suffice. The large and stable patient base ensures sustained procedure volumes. This provides a predictable market foundation. The ongoing burden of disease highlights the need for effective, minimally invasive interventions. These factors together create a long-term growth opportunity for sinus dilation devices.
2. Shift to Office-Based Care
There is a strong shift from hospital operating rooms to office-based procedures. Balloon sinus dilation under local anesthesia is showing strong outcomes. Patients benefit from less downtime, faster recovery, and reduced overall costs. Physicians also benefit from workflow efficiency. Studies report sustained symptom relief and low revision rates in patients treated in the office. Moving care into the clinic environment expands patient access and reduces reliance on higher-cost hospital settings. This makes treatment more affordable and convenient. The trend toward office-based care is expected to accelerate adoption and broaden the reach of sinus dilation devices.
3. Favorable Comparative Outcomes
Clinical evidence supports balloon dilation as an effective option for selected patients. Meta-analyses show fewer complications and shorter procedure times compared to traditional functional endoscopic sinus surgery (FESS). These results highlight balloon dilation as both safer and more efficient in appropriate cases. Providers benefit from improved quality metrics, while patients gain quicker relief. This quality and efficiency story is valuable for hospitals, clinics, and insurers. The evidence base continues to grow, strengthening the position of balloon dilation as a first-line minimally invasive solution. Favorable comparative outcomes make this an attractive choice in sinus care.
4. Health-Economic Upside
The economic case for balloon dilation is increasingly strong. Studies show that when lost productivity, missed workdays, and broader social costs are considered, balloon dilation is more cost-effective than many surgical alternatives. Faster recovery and fewer complications contribute to savings for both patients and payers. Real-world analyses confirm these benefits across diverse healthcare settings. This aligns with the move toward value-based care models. Balloon dilation’s ability to demonstrate savings beyond the procedure itself supports reimbursement negotiations. The health-economic upside creates a compelling case for broader adoption by payers, employers, and healthcare systems.
5. Professional Support for Coverage
Professional endorsement is a powerful driver of market access. The American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS) has issued supportive statements. These encourage insurance coverage of balloon dilation as a standard therapeutic tool. Alignment with such respected professional guidance helps reduce payer resistance. It also reassures providers about the legitimacy of the procedure. Support from medical societies strengthens credibility and encourages guideline inclusion. This, in turn, facilitates faster adoption by clinicians. Strong professional backing provides a solid foundation for market growth, while enabling stakeholders to engage in more effective access discussions with payers.
6. Technology and Safety Improvements
Technological advances are making balloon dilation safer and more standardized. Image-guided systems, structured training, and clear protocols reduce risks of rare but severe complications. Continuous improvement in device design further enhances reliability. Publishing real-world outcomes and registry data is building trust among clinicians. Safety improvements reassure payers, patients, and regulators alike. The combination of innovation and training strengthens clinical confidence and supports broader adoption. As complications decrease, the procedure becomes more attractive across healthcare systems. Ongoing advancements in technology and evidence collection will continue to drive growth and secure the role of balloon dilation devices.
7. International Expansion
Sinus disease prevalence is not limited to the U.S. Epidemiology studies confirm a comparable burden of rhinosinusitis across Europe and Asia, including China. This presents strong global expansion opportunities. Adoption, however, depends on reimbursement systems, physician training, and regulatory pathways in each region. With growing healthcare investment worldwide, the potential market is significant. Tailoring strategies to local needs can unlock new revenue streams. Companies that focus on education, partnerships, and regional access will be best positioned. International expansion represents one of the most promising long-term growth opportunities for sinus dilation devices beyond the U.S. market.
Conclusion
The sinus dilation devices market is set for steady growth, supported by rising cases of sinus-related disorders and the growing preference for minimally invasive treatments. Balloon dilation has gained wide acceptance due to its safety, effectiveness, and faster recovery outcomes. Favorable policies, reimbursement support, and continued device innovations are making adoption easier across healthcare systems. Despite challenges such as high costs, regulatory oversight, and payer scrutiny, the outlook remains positive. Expanding elderly populations, stronger global awareness, and the shift toward office-based care provide clear opportunities for further market penetration. With leading players driving innovation, the market is expected to remain highly dynamic and competitive.
View More
ENT Devices Market || Sinus Therapeutic Drug Market || Endoscopic Closure Systems Market || Ambulatory Surgical Center Market || US Ambulatory Surgical Center Market || Allergy Diagnostics and Therapeutics Market || Food Allergy Treatment Market || Allergy Immunotherapy Market
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



