Table of Contents
Overview
New York, NY – Aug 11, 2025: Global RNA-Based Therapeutics Market is projected to grow from USD 8.1 Billion in 2024 to USD 41.3 Billion by 2034. This represents a strong CAGR of 17.7% over the forecast period. One major factor driving this growth is the urgent global need for more precise treatments. RNA therapies offer targeted action by either blocking harmful genes or producing beneficial proteins. These therapies show great promise in tackling complex diseases like cancer and genetic disorders, where traditional drugs often fall short.
Government support is also boosting the market. Public health agencies such as the U.S. Department of Health and Human Services are actively funding RNA research. These efforts aim to strengthen pandemic preparedness and improve vaccine development. Other global health authorities are backing similar programs, recognizing RNA’s ability to speed up vaccine and therapeutic production. These initiatives create a strong foundation for future innovations and increase confidence in RNA technologies.
Technological progress is playing a critical role as well. Improved delivery systems, like lipid nanoparticles, help protect RNA molecules and ensure they reach the right cells in the body. These systems make treatments more effective and reduce potential side effects. Moreover, researchers are developing new RNA formats that are more stable and work faster, improving outcomes and allowing more flexibility in treatment design.
Regulatory agencies are also making it easier for RNA drugs to enter the market. The U.S. FDA and the European Medicines Agency have introduced fast-track approval pathways. These allow quicker evaluation of RNA-based drugs, especially for rare or life-threatening conditions. This faster approval helps companies bring treatments to patients sooner, encouraging more investment and innovation in this space.
Finally, global interest in RNA therapeutics is growing. While North America and Europe continue to lead in research, countries in Asia are increasing their investment in RNA-based technologies. New research centers and clinical trials are emerging across the region. Public-private partnerships are also gaining momentum, as governments collaborate with biotech firms and universities. These partnerships support shared development and have already led to several breakthrough RNA treatments and vaccines.
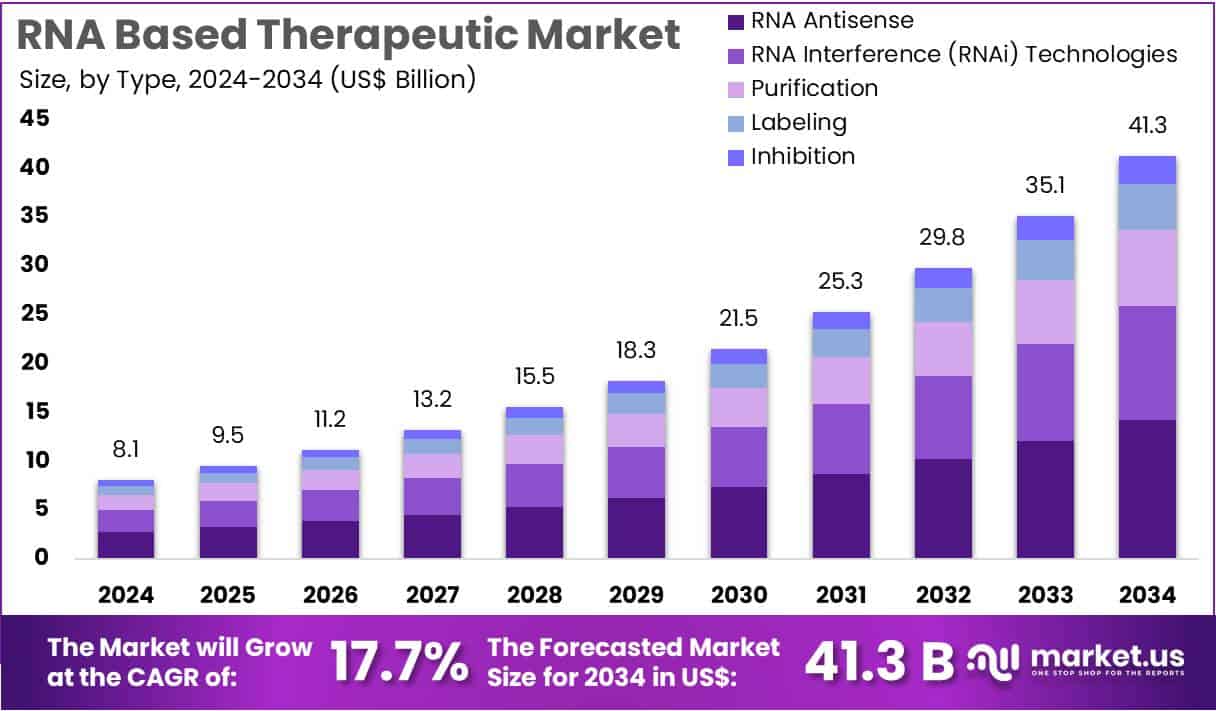
Key Takeaways
- In 2024, the RNA-based therapeutic market reached a revenue of US$ 8.1 billion, with strong growth projected at a 17.7% CAGR.
- By 2034, the market is forecasted to hit US$ 41.3 billion, driven by innovation and expanding medical applications of RNA therapies.
- RNA antisense technology led the type segment in 2024, capturing a dominant market share of 34.5% among other RNA-based tools.
- Genetic disorders accounted for the largest application share, representing 38.2% of the total market due to increasing focus on rare disease treatments.
- Research institutes dominated the end-user segment, holding a notable 45.8% share, reflecting their crucial role in therapeutic development and innovation.
- North America emerged as the leading region, contributing 37.8% of the global RNA-based therapeutic revenue in 2024 due to advanced R&D infrastructure.
Segmentation Analysis
- Type Analysis: RNA antisense is set to lead the RNA-based therapeutic market with a 34.5% market share. This method uses synthetic RNA to bind and silence target mRNA, helping to treat genetic and viral diseases. Strong clinical trial results, especially for spinal muscular atrophy and Duchenne muscular dystrophy, are fueling growth. These therapies allow precise gene silencing, reducing side effects. As regulatory bodies approve more antisense treatments, investments in this field are rising. More companies are developing targeted antisense therapies for various disorders, boosting the segment’s future potential.
- Application Analysis: Genetic disorders are the top application area in RNA-based therapeutics, holding 38.2% of the market. Their rising global prevalence is increasing the demand for advanced treatments. RNA-based approaches like antisense and RNAi offer new ways to treat rare and complex genetic diseases. These therapies can fix genetic errors at the mRNA level, making them effective and targeted. With ongoing innovations in gene editing and precision medicine, the use of RNA therapies for genetic conditions is expected to grow. This trend is reshaping treatment strategies worldwide.
- End-User Analysis: Research institutes dominate as end users in the RNA-based therapeutics market, holding 45.8% of the share. They drive innovation by advancing RNAi and antisense technologies. Supported by public and private funding, these institutes lead in drug discovery and disease modeling. Their efforts help improve RNA biology understanding and therapeutic use. Collaborations with biotech firms and pharmaceutical companies are also growing. These partnerships accelerate development timelines. As research deepens, institutes will continue expanding their role in RNA-based treatments, boosting the segment’s influence and long-term value.
Regional Analysis
North America leads the RNA-based therapeutic market
North America dominated the RNA-based therapeutic market with a 37.8% revenue share. This leadership is driven by robust R&D activity, accelerated regulatory approvals, and heavy investments from top pharmaceutical companies. By April 2024, the U.S. FDA had approved over 21 RNA-based treatments, including mRNA vaccines and RNAi therapies. Pfizer generated US$ 3.4 billion from Comirnaty in Q4 2024, while Moderna reported US$ 3.1 billion in full-year sales of Spikevax. These milestones highlight North America’s continued strength in RNA medicine development and commercialization.
Asia Pacific emerges as the fastest-growing region
Asia Pacific is projected to grow at the highest CAGR in the RNA therapeutics market. The region benefits from increased government backing for biotech innovation and a rising burden of treatable diseases. In 2024, China’s R&D spending exceeded CNY 3.6 trillion (about US$ 0.5 trillion), with a strong emphasis on biopharma. Japan’s AMED is also boosting RNA drug research and regenerative therapies. International players are expanding their presence here. Moderna’s global sales, including Asia Pacific, reached US$ 679 million in Q4 2024, signaling strong regional growth potential.
Key Players Analysis
Key players in the RNA-based therapeutics market use various strategies to boost growth. They focus on expanding their product lines with new treatments for genetic disorders, cancers, and infectious diseases. Investment in research and development is a top priority to improve the safety and effectiveness of their therapies. Companies also form strategic alliances with biotech firms, research centers, and healthcare providers. These partnerships speed up innovation and support the clinical adoption of RNA therapies. Many players are also building strong global supply chains to meet increasing demand.
Ionis Pharmaceuticals is a leading name in RNA-based therapeutics. Based in Carlsbad, California, Ionis develops RNA-targeted therapies and has three approved drugs: Spinraza, Tegsedi, and Waylivra. It also has four pipeline drugs in late-stage trials for diseases like Huntington’s, ALS, and cardiovascular conditions. The company’s proprietary platform improves both safety and effectiveness. Strategic partnerships with large pharmaceutical firms further support its pipeline growth. Ionis’s consistent trial success has made it a key driver in the RNA therapeutics market.
Emerging Trends
- RNA Editing Is Becoming More Common: New RNA-based therapies now focus on editing RNA instead of DNA. This method allows scientists to fix faulty gene messages without changing the genetic code permanently. Because the changes are temporary, the treatments are safer and reversible. This is helpful for testing and adjusting treatments as needed. Several biotech companies have already begun clinical trials using RNA editing. These trials focus on rare genetic disorders that currently have few or no treatments. The temporary nature of RNA editing makes it a flexible tool. It allows doctors to stop or adjust treatment if side effects occur.
- New Types of RNA Are Being Explored: Researchers are discovering new types of RNA for advanced treatments. Beyond mRNA, scientists are testing self-amplifying RNA, which can make copies of itself inside cells. This may reduce the dosage needed. Small activating RNA is another promising type. It works by turning on helpful genes rather than turning off harmful ones. Circular RNA is also gaining attention because it stays longer in the body. This increases the chance of better results from a single dose. These new RNA types offer longer-lasting effects, lower side effects, and improved effectiveness in treating diseases.
- Artificial Intelligence Is Helping Design Better RNA: AI is playing a key role in the development of RNA therapies. Machine learning tools can quickly study and analyze thousands of RNA sequences. These programs help scientists predict which sequences will be most stable and effective. AI also reduces the risk of side effects by identifying harmful patterns early. This speeds up drug discovery and reduces costs. Developers are now using AI to design personalized RNA treatments. These are made to match a patient’s unique genetic profile. With AI, the future of RNA medicine is faster, safer, and more accurate.
- Faster and Flexible Manufacturing Is on the Rise: Manufacturing RNA therapies used to be slow and expensive. But new technology is changing that. Companies can now produce RNA in smaller, flexible batches. This helps in treating rare diseases or customizing therapies for individual patients. Faster production means patients don’t have to wait long for treatment. This is especially helpful in cancer care, where time is critical. New tools also lower production costs. As a result, RNA treatments are becoming more accessible. Personalized and rare disease therapies are now easier to deliver thanks to these innovations in manufacturing.
Use Cases
- Heart and Blood Vessel Diseases: RNA-based treatments are now used to support heart health. These therapies help lower harmful fats like LDL cholesterol in the blood. They also work to improve heart muscle function. Some RNA drugs target faulty gene instructions that can lead to heart failure or stroke. This is a big step for people who don’t respond well to standard heart medications. RNA therapy may offer long-term benefits with fewer doses. Early clinical results show improvements in blood pressure and artery health. These treatments are now being tested in patients at high risk of heart disease.
- Genetic Disorders: RNA therapy is changing how we treat rare genetic diseases. These disorders are caused by faulty genes that give the wrong instructions to cells. RNA-based treatments can block or fix those harmful messages. This stops the disease from progressing. Conditions like spinal muscular atrophy and Duchenne muscular dystrophy are already seeing success. These patients often had no effective treatments before. RNA therapies are giving new hope to families affected by rare conditions. Trials are expanding to cover more genetic illnesses. Doctors believe these treatments may eventually become standard care.
- Brain and Nerve Conditions: Researchers are testing RNA therapies for diseases that affect the brain and nerves. This includes conditions like Huntington’s disease, ALS, and certain muscular dystrophies. These illnesses often get worse over time. RNA treatments aim to slow or stop that process. They do this by silencing faulty gene activity that causes nerve damage. The goal is to protect brain and nerve cells from further harm. Early studies show RNA therapies may reduce symptoms and improve quality of life. If successful, these treatments could offer new options where few exist today.
- Eye Diseases: RNA-based therapies are being used to treat serious eye conditions. These treatments focus on slowing vision loss caused by genetic or age-related problems. In diseases like macular degeneration, RNA can block harmful proteins that damage the retina. By stopping these proteins, the therapy helps protect vision. Some treatments are injected directly into the eye. Others may be given systemically. Early results from clinical trials are positive. Patients have shown slower disease progression and better vision preservation. RNA therapy may soon become a standard treatment for chronic eye disorders.
Conclusion
In conclusion, RNA-based therapeutics are shaping the future of medicine by offering more targeted and effective treatments for complex diseases. These therapies are gaining global attention due to their ability to treat conditions that were previously hard to manage. Backed by government support, strong research efforts, and faster regulatory approvals, the RNA field is moving forward quickly.
Advances in technology and artificial intelligence are also helping make these treatments safer, more affordable, and easier to produce. As more RNA therapies enter clinical use, patients with genetic, heart, brain, and eye conditions may soon have better treatment options. The growing interest and innovation in RNA suggest a bright future for this field.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



