Table of Contents
Introduction
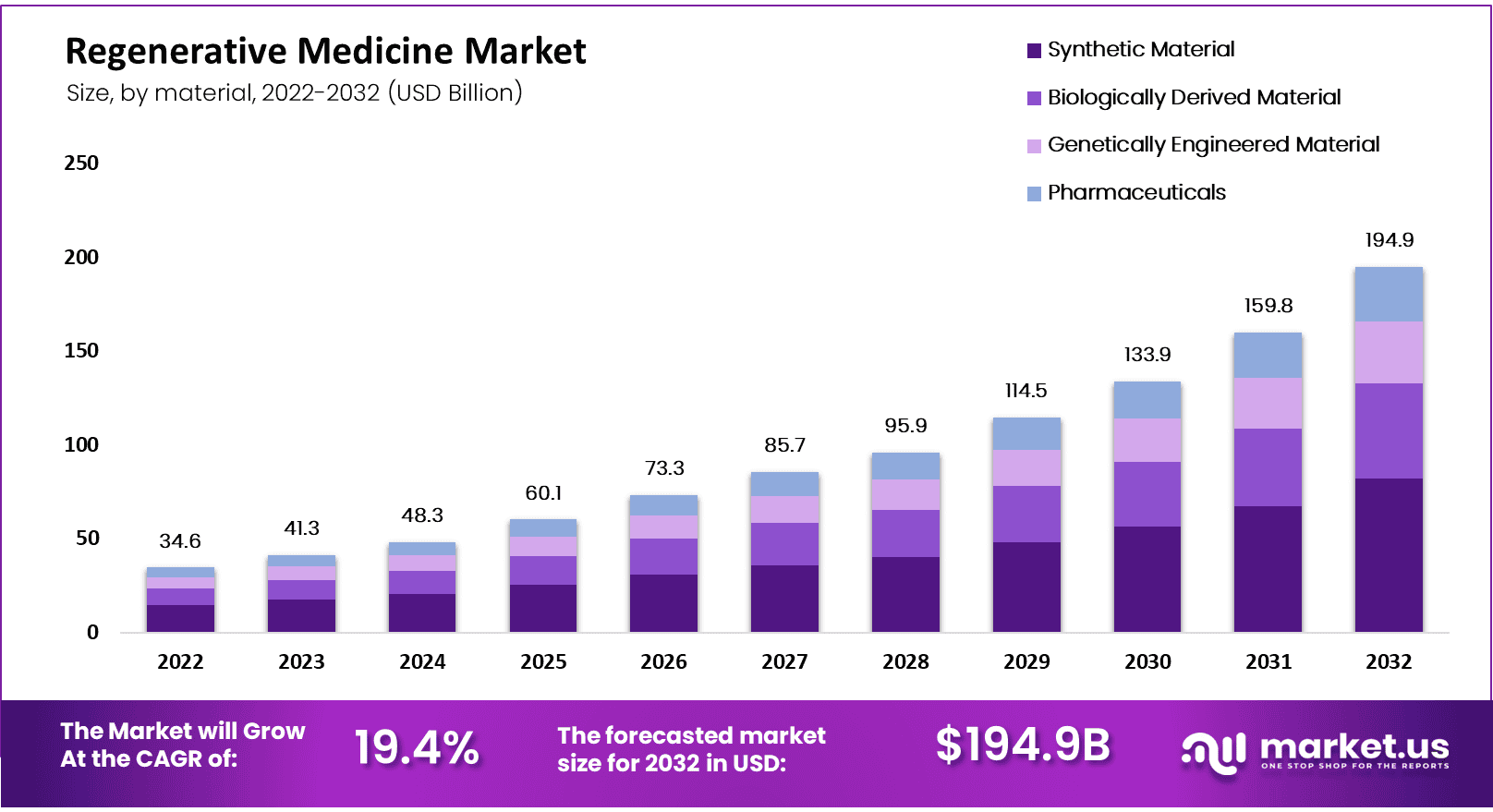
Global Regenerative Medicine Market size is expected to be worth around USD 194.9 Billion by 2032 from USD 41.3 Billion in 2023, growing at a CAGR of 19.40% during the forecast period from 2023 to 2032. In 2023, North America led the market, achieving over 52% share with a revenue of US$ 19 Billion.
This growth is primarily driven by the rising prevalence of chronic diseases like diabetes, cancer, and cardiovascular disorders, which increases the need for advanced treatments. Key advancements in stem cell therapy, gene therapy, and tissue engineering are at the forefront of this growth. Innovations such as artificial organs and lab-on-a-chip technologies have improved disease understanding, drug testing, and therapeutic methods.
Government support and favorable regulatory policies significantly contribute to market expansion. Regulatory frameworks ensure the safety and efficacy of regenerative therapies while promoting innovation. Additionally, public and private funding bolsters research and development, with significant investments in areas like cancer and neurological studies driving progress. For instance, the FDA’s policy framework facilitates the development and approval of new therapies while maintaining rigorous safety standards.
Collaborations between companies and research institutions further boost the market, enabling resource sharing and faster development of advanced treatments. Partnerships in stem cell research and gene therapy are paving the way for innovative therapies for various conditions.
Recent industry developments emphasize the potential of regenerative medicine. In June 2022, Bristol Myers Squibb received FDA approval for its CAR T-cell therapy, Breyanzi, designed to treat large B-cell lymphoma, a type of cancer. This milestone highlights the transformative potential of CAR T-cell therapies, which modify a patient’s T cells to target cancer cells.
Additionally, Avista Therapeutics partnered with F. Hoffmann-La Roche Ltd in the same month to develop an advanced adeno-associated virus (AAV) gene therapy vector for eye diseases. This collaboration leverages Avista’s single-cell AAV engineering platform to create targeted treatments for ocular conditions. With Roche committing up to $1 billion based on milestones, this partnership underscores the industry’s focus on addressing previously untreatable diseases.
Key Takeaways
- Market Valuation by 2032: The regenerative medicine market is anticipated to reach approximately USD 194.9 billion by 2032.
- Market Size in 2022: In 2022, the market size was around USD 34.6 billion.
- Growth Rate Projection: The market is projected to grow at a CAGR of 19.4% annually from 2023 to 2032.
- Shift to Biological Therapies: Biological therapies have gained popularity over traditional treatments.
- Impact of COVID-19: The COVID-19 pandemic has affected the delivery of CAR T-cell therapy and research activities.
- Chronic Disease Demand: Chronic diseases like cancer, diabetes, and heart disease drive the demand for regenerative medicine.
- Advancements in Stem Cell Research: Stem cell research significantly contributes to the growth of regenerative medicine.
- Aging Population Influence: The aging population leads to increased demand for regenerative medicine.
- Increase in Government Funding: Government funding for regenerative medicine research is on the rise.
- Cost as a Barrier: The high cost of regenerative medicine procedures can hinder adoption.
Regenerative Medicine Statistics
- Clinical Trials:
- Currently, there are 1,220 active clinical trials in regenerative medicine, including 685 in Phase 1, 383 in Phase 2, and 152 in Phase 3.
- Oncology dominates with 554 trials, followed by 94 for central nervous system (CNS) disorders and 87 for infectious diseases.
- FDA Approvals and Impact:
- By 2030, the FDA is anticipated to approve around 60 new gene, cell, and tissue therapies, benefiting over 500,000 people in the US.
- Multiple Myeloma:
- A total of 186 regenerative therapies for multiple myeloma are in development, with 74 in clinical trials and 117 developers involved.
- Healthcare costs for multiple myeloma patients average $280,000 annually, with a five-year survival rate following diagnosis.
- Bone Healing and BMP-2:
- Approximately 5%–10% of bone fractures result in non-unions.
- Recombinant human BMP-2 (rhBMP-2) is widely used in the US and Europe, with significant off-label applications for non-union treatments.
- Transgenic BMP-2 is 100 times more effective in signaling than rhBMP-2, with over 30% remaining cell-associated when delivered via adenovirus.
- Therapeutic Innovations:
- Breakthroughs in gene therapy, gene editing, and cell therapy are addressing the root causes of diseases.
- COVID-19 Challenges:
- While the pandemic disrupted clinical trials, it also accelerated innovation and reduced development timelines.
- Global Clinical Trials and Market Projections
- FDA Approvals in 2022:
- The FDA approved over 15 regenerative medicine products.
- Patient Outcomes:
- Globally, regenerative therapies have been used to treat approximately 300,000 patients.
- Investment:
- Investments in regenerative medicine exceeded $10 billion in 2021.
- Stem Cell Therapy:
- Stem cells have been shown to reduce the risk of heart attack by 58% and stroke by 75% in patients with high inflammation.
- Autologous stem cell transplants decreased disability in 19% of multiple sclerosis patients over five years.
- A robotic system being tested in the UK aims to improve stem cell manufacturing by lowering costs and minimizing errors.
- COVID-19 and Stem Cells:
- Stem cell therapy improved survival rates in COVID-19 patients by 2.5 times and significantly reduced inflammation within 14 days.
- Lung function improved in 75% of treated patients, with 45% experiencing reduced ventilator dependence.
- Clinical trials for stem cell therapy during the pandemic included 210 patients across multiple centers.
Emerging Trends in Regenerative Medicine
- Personalized Medicine: The use of a patient’s own cells to create personalized organs and tissues is gaining traction. This approach minimizes the risk of organ rejection and improves treatment efficacy by tailoring therapies to individual genetic profiles. Autologous cell therapy techniques are being refined to enhance patient outcomes.
- Gene Therapy Advancements: Progress in viral and non-viral vector development is enabling precise gene delivery, expanding the applications of gene therapy. These advancements allow for targeting and correcting genetic mutations responsible for rare genetic diseases and certain cancers.
- Stem Cell Research: Advances in pluripotent stem cell research are opening new therapeutic possibilities for conditions like diabetes, Parkinson’s disease, and heart disease. These cells, capable of differentiating into various cell types, offer potential solutions for regenerating damaged tissues and treating previously untreatable diseases.
- Tissue Engineering: Innovations in tissue engineering are driving the creation of functional tissues and organs, including artificial skin, cartilage, and complex organs. These breakthroughs are moving closer to clinical use, offering hope for patients with severe tissue damage or loss.
- Regulatory Support: Regulatory bodies like the FDA have implemented policies to support regenerative medicine, streamlining approval processes, encouraging innovation, and ensuring patient safety. These efforts are accelerating the development of life-changing therapies.
- Public-Private Partnerships: Collaborations between government entities, academic institutions, and private companies are reducing costs and fostering innovation in regenerative medicine. These partnerships are critical for overcoming technical challenges and expediting the development of new treatments.
- Standardization Efforts: Industry-wide initiatives to establish standards for regenerative medicine products are underway. Standardization ensures the safety, quality, and consistency of therapies, building trust among stakeholders and facilitating regulatory approvals.
- Investment in R&D: Substantial investments from both government and private sectors are fueling research and development, leading to the discovery of novel regenerative therapies, enhancement of existing treatments, and commercialization of cutting-edge technologies.
Use Cases in Regenerative Medicine
- Cardiac Regeneration: Regenerative therapies are being developed to repair heart tissues damaged by heart attacks. Stem cell-based treatments aim to regenerate heart muscle, improve blood vessel formation, and enhance cardiac function, potentially reducing heart failure risks.
- Diabetes Management: Research is focusing on regenerating insulin-producing beta cells in the pancreas using stem cells. Innovations include bioengineered pancreatic islets and gene therapies to restore insulin production and regulate blood glucose levels, offering hope for type 1 diabetes patients.
- Osteoarthritis Treatment: Regenerative techniques, such as stem cell injections and cartilage tissue engineering, aim to repair damaged joints. These approaches focus on alleviating pain, reducing inflammation, and restoring joint function, potentially delaying or eliminating the need for joint replacement surgery.
- Neurological Disorders: Therapies for conditions like Parkinson’s disease, multiple sclerosis, and spinal cord injuries are being developed to replace damaged neurons, promote nerve regeneration, and restore lost functions. Neuroprotective strategies are also being researched to slow disease progression.
- Liver Regeneration: Innovative methods using stem cells and bioengineered liver constructs are being explored to treat liver diseases like cirrhosis and hepatitis. These approaches aim to regenerate liver tissue, potentially reducing the need for transplants.
- Wound Healing: Advances in bioengineered skin grafts, growth factors, and cellular therapies are transforming wound care. These treatments are designed to accelerate the healing of chronic wounds, such as diabetic ulcers and severe burns, by promoting tissue regeneration.
- Cancer Treatment: CAR T-cell therapy represents a breakthrough in cancer care by modifying a patient’s T cells to target and destroy cancer cells. Ongoing research aims to expand its application to solid tumors, enhancing specificity and minimizing side effects.
- Eye Diseases: Gene therapies targeting retinal diseases, including retinitis pigmentosa and age-related macular degeneration, are being developed. These therapies aim to restore vision by repairing or introducing genes crucial for retinal health, potentially reversing vision loss.
Conclusion
Regenerative medicine is rapidly transforming healthcare through groundbreaking advancements in stem cell therapy, gene therapy, and tissue engineering. With rising prevalence of chronic diseases and aging populations driving demand, innovations like personalized medicine and CAR T-cell therapies are addressing unmet medical needs. Regulatory support, public-private partnerships, and substantial investments are accelerating research, enhancing therapeutic efficacy, and expanding market reach.
Despite challenges like high costs and COVID-19 disruptions, the field shows immense promise in treating diseases previously considered incurable. By 2032, the market is projected to grow exponentially, offering hope for improved patient outcomes and ushering in a new era of precision medicine.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



