Table of Contents
Overview
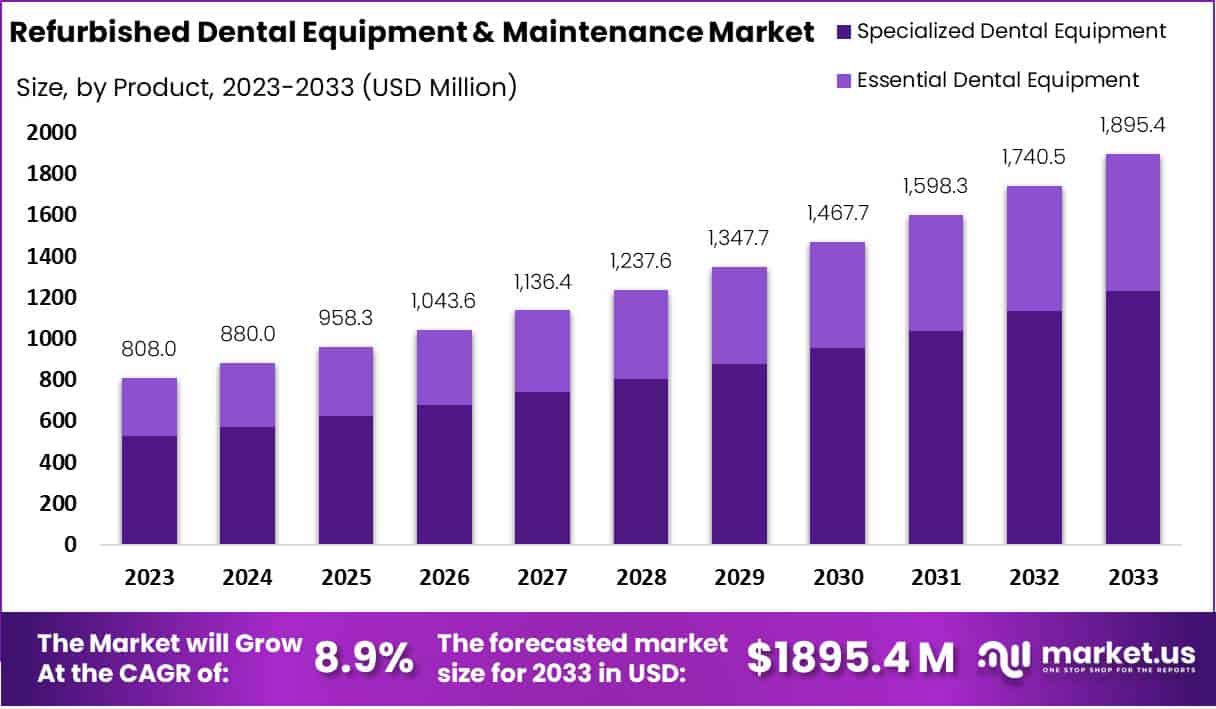
The global Refurbished Dental Equipment & Maintenance Market was valued at USD 808 million in 2023 and is projected to reach USD 1,895.4 million by 2033, growing at a CAGR of 8.9% from 2024 to 2033. This growth is driven by the increasing oral disease burden, cost-efficiency needs in healthcare systems, and the expansion of sustainable procurement models. According to the World Health Organization (WHO), oral diseases affect nearly 3.5 billion people worldwide, highlighting a vast and sustained demand for dental care equipment.
A WHO Global Oral Health Status Report (2022) reveals that around 2.5 billion individuals suffer from untreated dental caries, while 7% of people aged 20 years and above experience complete tooth loss, increasing to 23% in those over 60 years. In the United States alone, 46% of children aged 2–19 years have untreated or restored caries, and 13.2% of adults aged 65+ experience full tooth loss. These statistics indicate an unmet need for dental care, supporting long-term demand for both new and refurbished dental equipment globally.
The economic burden of oral diseases remains substantial. In 2019, global costs associated with oral conditions were estimated at USD 710 billion, including USD 387 billion in direct treatment costs and USD 323 billion in productivity losses. Such high expenses push healthcare providers toward cost-effective alternatives like refurbished equipment. For instance, about 20–30% of U.S. hospitals report reusing at least one type of single-use medical device, showing openness toward safe and validated reuse models. The same cost-saving logic is influencing dental clinics worldwide to adopt refurbished tools.
Furthermore, regulatory clarity has strengthened trust in refurbished equipment. The U.S. Food and Drug Administration (FDA) has issued guidelines for the validation, reprocessing, and labeling of reprocessed medical devices. Similar frameworks in other regions differentiate between refurbishment and remanufacturing, reducing uncertainty for suppliers and buyers. These regulations enhance transparency, promoting safety and quality assurance in refurbished dental devices, and fostering a favorable environment for market growth.
Maintenance, Sustainability, and Policy Influence
Infection control standards continue to drive the need for regular maintenance and replacement of dental instruments. According to the Centers for Disease Control and Prevention (CDC), all dental handpieces must undergo heat sterilization between uses. Over time, these repeated sterilization cycles degrade components, necessitating periodic servicing, parts replacement, and quality validation. This ensures compliance with infection control protocols and sustains steady demand for equipment maintenance services.
Public health initiatives and universal health coverage programs are also supporting market expansion. The World Health Assembly Resolution WHA74.5 (2021) urged countries to integrate oral health into primary healthcare systems. As a result, several nations are scaling up dental service access. For example, England’s NHS delivered 35 million dental treatment courses in 2024–25, marking a 4% year-on-year increase. These expansions push procurement departments to opt for cost-efficient solutions, including refurbished dental equipment.
Environmental sustainability is another key growth driver. Concerns over rising medical waste and e-waste are prompting organizations to favor refurbishment. The WHO has raised alarms about the dangers of informal e-waste recycling, especially for vulnerable populations. Refurbishing and reusing dental equipment extend the lifecycle of devices, reducing waste and aligning with global sustainability targets. Governments and donors are increasingly adopting “green procurement” models that prioritize eco-friendly and cost-effective equipment options.
Secondary markets further support the availability of refurbished dental equipment. When clinics or hospitals upgrade their systems, older yet functional equipment often re-enters the market through validated refurbishment processes. These refurbished devices are then redeployed in smaller clinics or cost-sensitive regions. According to industry estimates, the Refurbished Dental Equipment & Maintenance Market is poised to grow at a steady 8.9% CAGR, reaffirming its critical role in affordable, sustainable, and accessible dental care worldwide.
Key Takeaways
- The refurbished dental equipment and maintenance market is projected to reach USD 1,895.4 million by 2033, growing at a CAGR of 8.9%.
- Refurbished dental equipment provides 50–60% cost savings compared to new products, offering vital affordability for budget-conscious dental practices worldwide.
- In 2023, the Specialized Dental Equipment segment held a 65% market share, supported by sustainability initiatives and cost-efficient technological solutions.
- Hospitals accounted for over 43% of the 2023 market, preferring refurbished equipment to maintain a balance between innovation and affordability.
- Refurbished devices offer multiple benefits, including lower initial investment, reduced energy consumption, and 2–3 times higher return on investment (ROI).
- Market growth is restrained by quality and reliability concerns, as 30% of practitioners remain cautious due to perceived patient safety risks.
- North America led globally in 2023, capturing a 47.7% share worth USD 385.4 million, driven by advanced healthcare infrastructure and digital adoption.
- The Asia-Pacific region is experiencing rapid expansion, fueled by rising dental awareness, increased healthcare spending, and growing dental service availability.
- Dental tourism in developing countries presents a major opportunity, where refurbished equipment ensures affordability and global quality compliance.
- The digital dentistry trend is accelerating, with 35% adoption of digital tools, boosting demand for refurbished digital dental equipment.
Regional Analysis
In 2023, North America dominated the Refurbished Dental Equipment & Maintenance Market with a 47.7% share, valued at USD 385.4 million. This dominance is due to advanced healthcare infrastructure and high adoption of digital dentistry. The presence of key market players strengthens regional leadership. Strict regulations on dental equipment quality and maintenance ensure equipment reliability and patient safety. The well-established refurbishment ecosystem drives consistent demand across hospitals, clinics, and private practices, making North America a benchmark for market standards and technological excellence.
Europe focuses on cost-effective dental solutions due to rising healthcare expenses. Many small and medium-sized dental practices in the region prefer refurbished equipment to manage operational budgets efficiently. The shift toward sustainable healthcare practices also supports refurbishment. Countries like Germany, France, and the U.K. show steady adoption driven by quality assurance programs and equipment certification standards. This regional emphasis on affordability and eco-friendly operations sustains market growth, positioning Europe as a stable but value-driven market for refurbished dental systems.
The Asia-Pacific region is experiencing rapid growth, supported by increasing dental awareness and healthcare spending. Expanding dental services in China, India, and Southeast Asia encourage adoption of refurbished equipment. The growing middle class and rising dental tourism further contribute to market expansion. Emerging economies prioritize cost efficiency while improving access to dental care. Latin America and the Middle East & Africa, though smaller markets, show strong potential with ongoing healthcare infrastructure improvements and rising investments in dental technology and maintenance services.
Segmentation Analysis
Product Analysis
In 2023, the refurbished dental equipment market showed a clear shift, with the Specialized Dental Equipment segment capturing over 65% of the market share. This dominance was driven by its cost-effective offerings and advanced refurbishment processes. Dental practitioners preferred these tools for their quality and affordability, especially after the pandemic. The segment also benefited from sustainability trends, as professionals opted for eco-friendly solutions. Growing trust in refurbished technologies and innovations in digital systems and AI are expected to strengthen this segment’s market position further.
End User Analysis
In 2023, hospitals led the refurbished dental equipment market, accounting for more than 43% of the share. This growth reflected their focus on cost efficiency and quality patient care. Hospitals increasingly adopted refurbished equipment to meet regulatory standards while reducing capital spending. These investments supported both modernization and compliance goals. The demand for maintenance services and reliable technology reinforced this trend. As hospitals continue upgrading their dental facilities, their segment is projected to maintain leadership through prudent investments and operational optimization.
Key Players Analysis
The Refurbished Dental Equipment & Maintenance Market is shaped by several leading companies offering quality-driven and cost-effective solutions. American Dental Refurbishment is recognized for its extensive range of refurbished dental chairs, x-ray machines, and sterilization units. The company’s strict refurbishment standards ensure reliability and performance, helping dental practices maintain operational efficiency. Its growing presence in the North American market highlights the rising demand for affordable yet high-quality refurbished dental equipment across small and mid-sized dental clinics seeking practical alternatives to new installations.
Atlas Resell Management has gained prominence through its wide inventory and comprehensive refurbishment services. The company provides a vast selection of refurbished dental systems, meeting diverse client requirements. Its transparent reselling process and strong customer support have strengthened its position in the market. By focusing on quality assurance and after-sales service, Atlas Resell Management has developed a loyal customer base. This customer-centric approach ensures long-term partnerships and reinforces the company’s reputation as a trusted provider of refurbished dental solutions.
A & K Dental Equipment emphasizes premium refurbishment and durability, focusing on advanced dental technologies. The company specializes in refurbishing digital imaging systems and CAD/CAM units, aligning with the global shift toward digital dentistry. This focus has enhanced its competitiveness in high-tech dental markets. A & K’s commitment to technological precision and product reliability appeals to clinics aiming to upgrade their services cost-effectively. The brand’s attention to detail and continuous improvement strategy make it a preferred choice among technologically progressive dental facilities.
Capital Dental Equipment and other notable players, such as Collin’s Dental Equipment Inc., Independent Dental Inc., Pre-Owned Dental Inc., SPS Dental, Renew Digital LLC, and DCI Dental Equipment, contribute significantly to the market’s competitiveness. These companies specialize in digital radiography and imaging systems, helping clinics transition to digital platforms. Their combined efforts drive innovation, quality enhancement, and affordability within the refurbished dental equipment market. The presence of multiple specialized players ensures diverse offerings, promoting market growth and accessibility for dental practices worldwide.
Challenges
1. Regulatory Compliance and Documentation
Refurbished dental devices must follow strict regulations to avoid being classified as “remanufactured.” Once considered remanufactured, the device must meet full FDA requirements, including design control, labeling, and quality documentation. This increases the compliance workload for both sellers and buyers. In the European Union, refurbished or reprocessed medical devices must comply with the Medical Device Regulation (MDR). Each country may also apply its own rules for single-use device reprocessing. Failure to meet these standards can result in legal penalties and restrict market access for refurbished equipment.
2. Infection Control and Reprocessing Assurance
Dental tools such as handpieces must be sterilized after every use to prevent cross-contamination. Devices lacking validated cleaning and sterilization instructions should not be used. Refurbished handpieces must include verified reprocessing instructions and proof of compatibility with standard sterilization cycles. Additionally, dental unit waterlines must meet the EPA’s drinking-water standard of ≤500 CFU/mL. This requires ongoing treatment, cleaning, and testing. Refurbished dental chairs or units must support safe waterline management. Ensuring proper infection control is essential for patient safety and for maintaining regulatory compliance in dental practices.
3. Electrical Safety and Performance Testing
Regular safety testing is essential for refurbished dental devices. The IEC 62353 standard provides guidelines for electrical safety checks after servicing or repair. Buyers should request test reports that confirm compliance with this standard. These checks help prevent electrical hazards and maintain device performance over time. Refurbishers must ensure that all electrical components, cables, and connectors meet safety standards. Proper documentation of these tests builds trust between suppliers and clinics. Continuous performance verification also supports longer equipment life and reduces maintenance risks.
4. Parts Availability and Lifecycle Risk
Many older dental platforms face limited access to original parts and software updates. This increases the risk of equipment failure and regulatory non-compliance. Under EU MDR, a “fully refurbished” device must have a defined remaining useful life and a clear spare parts roadmap. Without this, clinics may face unexpected downtime or higher repair costs. Refurbishers should assess long-term part availability and provide documentation for lifecycle management. Predicting equipment longevity helps buyers make informed decisions and ensures continued patient safety and performance reliability.
5. Warranty, Liability, and Service Quality
Reliable warranties and transparent service histories are essential for reducing risk in refurbished dental equipment. Refurbishers should operate under ISO 13485-compliant quality systems to ensure consistent service and documentation. These measures improve accountability but can increase operational costs. Clinics benefit from warranties that clearly define coverage, response times, and service obligations. Traceable maintenance records also build buyer confidence. Proper liability management protects both refurbishers and dental professionals while ensuring patient safety and equipment reliability.
6. Perception and Procurement Barriers
Despite proven performance, refurbished dental devices are often perceived as lower quality. This perception limits adoption in clinics. Some procurement teams focus mainly on the lowest upfront cost rather than long-term value. However, refurbished equipment can deliver comparable results at lower lifecycle costs. Industry education, quality certifications, and transparent testing reports can help change buyer attitudes. Promoting environmental and economic benefits also supports market acceptance. Building trust through data-driven evidence is key to improving procurement confidence in refurbished devices.
7. Data Security and Software Updates (Digital Dentistry)
Modern dental systems like cone-beam CT scanners, CAD/CAM units, and sensors depend on secure software and data handling. Refurbished devices must support cybersecurity standards, licensing transfers, and software patching. Buyers and refurbishers should clarify responsibilities for updates and data protection. Altering software or digital functions may shift the device classification from “serviced” to “remanufactured,” requiring regulatory reassessment under FDA rules. Ensuring cybersecurity and licensing integrity protects patient data and device performance, especially as digital dentistry continues to expand rapidly.
8. Environmental Compliance for E-Waste
Improper disposal of medical and dental electronics contributes to the global e-waste problem. Refurbishers must establish responsible recycling and take-back programs to reduce environmental impact. Compliance with local and international e-waste regulations ensures safe material recovery and disposal. Refurbished device sellers should educate buyers on end-of-life management and provide documentation for sustainability practices. Promoting circular economy principles not only reduces waste but also strengthens brand reputation. Environmentally compliant refurbishment supports global efforts toward greener healthcare operations.
Opportunities
1. Cost Relief with Verified Quality
Refurbished dental systems help reduce costs without compromising safety or performance. These systems lower capital investment significantly compared to new equipment. When refurbishment follows strict documentation and testing standards, the devices meet required safety and efficiency benchmarks. Policy studies show that healthcare providers face constant pressure to manage costs while maintaining quality. Refurbished systems provide a practical solution. They offer value through lower acquisition costs, reliable operation, and compliance with performance regulations. This approach allows clinics to upgrade their equipment more frequently while maintaining financial control.
2. Sustainability and Circular Economy
Healthcare generates around 4.4–4.6% of global greenhouse gas emissions. Refurbishing medical and dental devices helps reduce this impact. Extending product lifecycles lowers embedded carbon emissions and supports the circular economy. Research shows refurbished CT scanners use about 32% less energy across their supply chain compared to new ones. This energy saving highlights the environmental value of refurbishment. By choosing refurbished systems, dental clinics contribute to sustainability goals while reducing waste. It supports responsible resource use and aligns with global sustainability commitments in healthcare operations.
3. Expanding Access in Cost-Sensitive Markets
In many low- and middle-income countries, access to dental equipment remains limited. Refurbished and well-documented devices help close this gap. They offer affordable and reliable options for clinics and hospitals with restricted budgets. By reusing certified systems, healthcare facilities can expand diagnostic and treatment capabilities without large financial burdens. This approach increases access to basic dental care and imaging services. As a result, more patients benefit from timely and effective treatments. Refurbishment supports equity in healthcare and strengthens local medical infrastructure in underserved regions.
4. Preventive and Predictive Maintenance Revenue
Standardized maintenance programs create new revenue streams for providers. Regular calibration, sterilizer monitoring, and electrical safety testing under IEC 62353 ensure long-term performance. Documentation aligned with ISO 13485 supports traceability and quality assurance. Clinics benefit from improved uptime and fewer unexpected breakdowns. These services also strengthen client trust and satisfaction. For refurbishers, offering preventive and predictive maintenance builds consistent income while enhancing brand credibility. The approach encourages long-term partnerships with clinics and promotes equipment reliability through continuous service support.
5. Infection-Control Services and Training
Ongoing infection control is critical in dental practices. Training programs on handpiece sterilization, waterline testing, and quality monitoring are essential. Refurbishers can provide these as repeatable service offerings. Clinics benefit from improved hygiene, compliance, and reduced infection risks. Proper documentation of testing and shock treatments builds confidence in device safety. By offering tailored infection-control training, refurbishers differentiate themselves from competitors. It also opens a recurring business opportunity. This integrated approach supports patient safety and strengthens the reputation of refurbished dental equipment providers.
6. Trade-In and Take-Back Programs
Trade-in and take-back programs help manage old dental equipment responsibly. Clinics can return used devices for credit or recycling. Certified data wiping ensures privacy protection for digital devices. Proper end-of-life recycling reduces electronic waste and meets environmental regulations. These programs also improve the perception of refurbished systems as safe and eco-friendly solutions. For suppliers, it creates a closed-loop system that builds customer loyalty and supports brand sustainability. Trade-in programs combine cost savings, compliance, and environmental stewardship for both providers and refurbishers.
Conclusion
The refurbished dental equipment and maintenance market is growing steadily due to the rising need for affordable, sustainable, and reliable dental solutions. Increasing oral health issues, cost pressures on healthcare systems, and a global shift toward eco-friendly practices are driving adoption. Refurbished devices help clinics maintain high-quality care while reducing costs and waste. Strong regulatory support and advances in refurbishment technology have improved trust and safety standards. As dental care expands in both developed and emerging regions, the demand for refurbished equipment and maintenance services will continue to rise, supporting accessible and sustainable dental healthcare worldwide.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



