Table of Contents
Overview
New York, NY – Aug 06, 2025 : The Global Preparative and Process Chromatography Market is projected to reach around US$ 28 Billion by 2034. It is growing from US$ 12.5 Billion in 2024, at a CAGR of 8.4% during 2025 to 2034. North America leads the market with a 32.2% share and holds a market value of US$ 4 billion in 2024. This region’s dominance is due to strong regulatory frameworks and advanced biopharma infrastructure. Increasing investments in biotech research continue to support market growth across the region and beyond.
Preparative and process chromatography is vital in purifying biopharmaceuticals. It helps isolate therapeutic proteins, vaccines, and monoclonal antibodies during downstream processing. These methods improve both yield and purity. As per BioProcess International, biosimilar production saw capture stage purity rise from 40% to 80%. Downstream yield improved from 11.3% to 33.4%. These gains show how innovation in purification enhances biopharma efficiency. The focus on high-purity biologics is driving industry-wide adoption of these techniques at every production level.
The demand for high-purity biologics fuels market expansion. As pharmaceutical companies prioritize monoclonal antibodies and recombinant proteins, efficient purification becomes critical. Preparative chromatography helps remove host cell proteins, DNA, and other unwanted particles. Yet, challenges remain. A PubMed study reveals nearly 20% of proteins may be lost at each purification step. This highlights the need for better optimization. The balance between high yield and purity continues to shape decisions in drug development and manufacturing.
Technology is transforming chromatography practices. Innovations like Multicolumn Countercurrent Solvent Gradient Purification (MCSGP) deliver up to 90% purity and 93% yield. These systems also offer tenfold productivity gains and reduce solvent use by 90%. Single-use chromatography systems are also gaining traction. They reduce contamination risks and make validation easier. These technological shifts not only improve efficiency but also cut operational costs. As pharmaceutical production scales up, advanced chromatography systems will become increasingly important for consistent quality output.
Regulatory compliance drives market standards. The U.S. FDA enforces CGMP guidelines and inspects labs every two years. CDER also monitors drugs for accuracy and impurities. Outside of pharma, chromatography helps ensure food safety. The FDA and CDC track foodborne pathogens, which cause 48 million illnesses annually in the U.S. Programs like FERN play a key role, despite recent funding cuts. With the rise of personalized medicine, the need for precise purification techniques will grow, boosting market demand further.
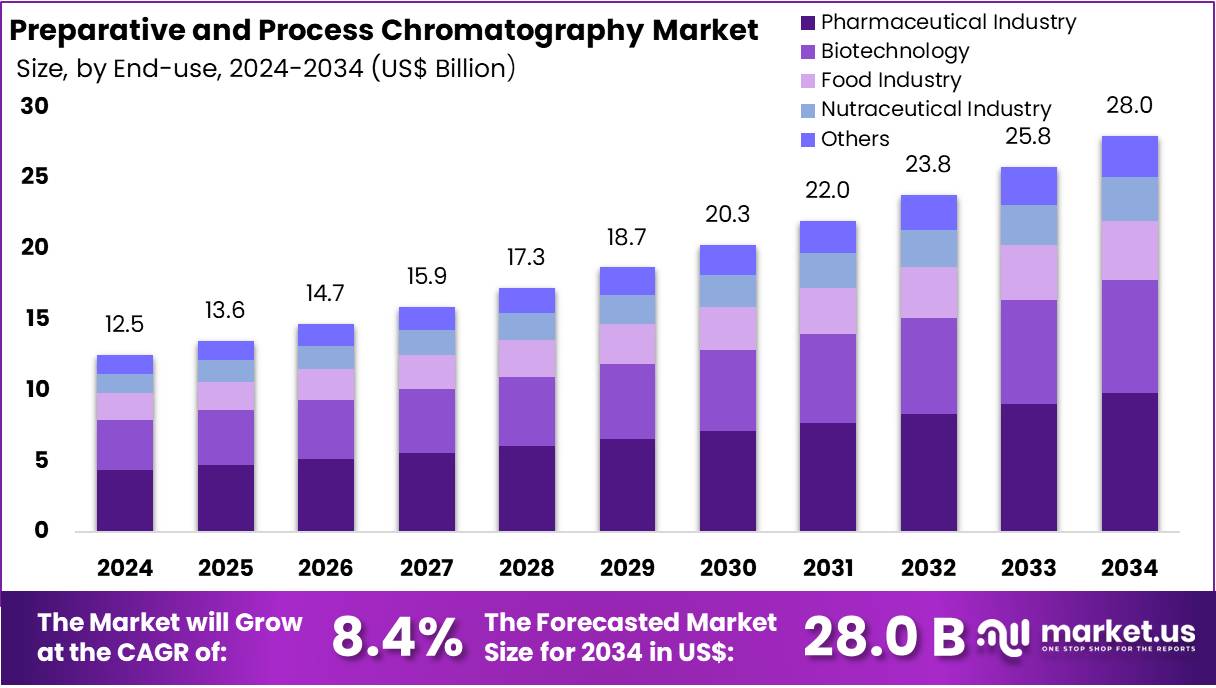
Key Takeaways
Massive Growth Ahead : An industry expert highlighted that the global preparative and process chromatography market is set to hit US$ 28 billion by 2034.
Strong CAGR Signals Market Strength : Between 2025 and 2034, the market is forecasted to grow at a healthy 8.4% CAGR, reflecting sustained industry demand and innovation.
Process Chromatography Leads Product Segments : In 2024, process chromatography led with over 59.2% market share, thanks to its ability to scale and handle complex separation tasks efficiently.
Liquid Chromatography Dominates by Type : Liquid chromatography claimed more than 28.4% of the global market share in 2024, making it the most widely adopted technique by type.
Pharma Industry Drives Majority Demand : With drug development on the rise, the pharmaceutical sector made up over 43.6% of total market demand in 2024.
North America Holds Top Regional Share : North America led globally in 2024, capturing around 32.2% of the market, valued at approximately US$ 4 billion.
Regional Analysis
In 2024, North America led the preparative and process chromatography market with over a 32.2% share, valued at US$ 4 billion. This dominance was supported by a strong pharmaceutical and biopharmaceutical base. Companies in the region invested heavily in drug development and purification systems. Advanced manufacturing, including large-scale molecule separation, became a key focus. Preparative and process chromatography tools were widely adopted in therapeutic workflows. These technologies played a vital role in ensuring quality and efficiency in drug production across the region.
The United States played a major role in driving North America’s growth. It benefited from the presence of leading biotech firms and contract manufacturers. Academic labs and research institutions in the U.S. regularly used chromatography tools for protein and compound purification. Supportive public funding and favorable regulations boosted adoption rates. Rising demand for biologics and gene therapies further pushed growth. Chromatography helped ensure purity in complex treatments. This trend is likely to continue as precision medicine advances and biologic manufacturing scales up.
Segmentation Analysis
In 2024, the Process Chromatography segment led the product category, capturing over 59.2% of the Preparative and Process Chromatography Market. This dominance came from its key role in large-scale purification. It is widely used to purify monoclonal antibodies, therapeutic proteins, and vaccines. These systems are ideal for high-volume, GMP-compliant production environments. Their scalability, regulatory support, and efficiency make them essential in pharmaceutical manufacturing. The rising demand for biologic drugs and continuous manufacturing processes further supports growth. Preparative chromatography also holds value, mainly in research labs and small-scale drug development.
In terms of type, Liquid Chromatography held the largest share at over 28.4% in 2024. It is known for accurate and reliable biomolecule separation. Its wide use in pharmaceutical development and purification strengthens its market position. Gas Chromatography is vital in food safety and petrochemical industries for volatile compound analysis. Thin Layer Chromatography remains common in academic settings due to its cost-effectiveness. Gel-permeation Chromatography and Hydrophobic Interaction Chromatography are also gaining traction. These are especially useful in protein separation, biopharma testing, and personalized drug applications.
The pharmaceutical industry led the market by end-use, accounting for more than 43.6% of the share in 2024. This was driven by rising biologics production and the need for high-purity APIs. Chromatography supports personalized medicine and meets strict regulatory demands. The biotechnology sector followed closely, using chromatography in genetic studies and protein purification. Academic and research institutions also expanded its use. In food and nutraceutical sectors, chromatography helps detect additives and extract active ingredients. Environmental labs and other users showed steady, application-specific adoption for chemical analysis and quality control.
Key Players Analysis
In 2024, North America led the global Preparative and Process Chromatography Market with over a 32.2% share, valued at around US$ 4 billion. This growth was fueled by a strong pharmaceutical and biopharmaceutical industry. Companies in the region heavily invested in drug development and purification technologies. They adopted advanced manufacturing processes, especially for large-scale molecule separation. Preparative and process chromatography became essential in therapeutic production. These systems were widely used to meet regulatory standards and improve efficiency across pharmaceutical operations in the region.
The United States was the main contributor to North America’s market growth. Key factors included the presence of major biotech firms and contract manufacturers. Research labs and academic institutions regularly used chromatography tools for purifying proteins and compounds. Supportive public funding and favorable regulations encouraged technology adoption. The rise in biologics, monoclonal antibodies, and gene therapies increased the need for high-purity production. As precision medicine grows, demand for advanced purification systems will rise. North America is likely to maintain its leadership position.
Emerging Trends
- Growing Focus on Biologic Drug Purification: Pharmaceutical companies are increasingly focusing on biologic drugs such as monoclonal antibodies and therapeutic proteins. These complex molecules require very high levels of purity, which is where preparative and process chromatography comes in. This technology is essential for separating and purifying biologics during production. As demand for these treatments grows, the need for advanced chromatography systems is rising. Manufacturers are investing in better purification tools to meet strict regulatory standards. Biologic drug development is also expanding into areas like cancer and autoimmune diseases, further driving this trend. As a result, chromatography is becoming a key part of modern drug development workflows.
- Increased Use in Continuous Manufacturing: The pharmaceutical industry is shifting from traditional batch processing to continuous manufacturing. This new approach helps companies produce drugs more efficiently and with less waste. Preparative and process chromatography systems are being redesigned to work in continuous environments. These modern systems allow steady purification of drug components without stopping production. As a result, companies can reduce overall production time and improve consistency in product quality. This trend is particularly strong in biologics and other high-value drugs. Continuous manufacturing also supports quicker response to market demands and regulatory changes, making it a smart investment for forward-looking pharmaceutical firms.
- Rise of Single-Use Technologies: Single-use technologies are becoming increasingly popular in chromatography applications. These include disposable columns, filters, and tubing used in purification processes. Companies prefer them because they save time, lower the risk of contamination, and reduce the need for cleaning. For small to medium-scale drug production, single-use systems offer flexibility and faster turnaround. They also help meet strict hygiene and safety standards. More facilities are now integrating disposable components, especially for clinical and pilot-scale batches. As biologics and personalized therapies grow, so does the demand for single-use options that support quick and efficient production cycles.
- Integration of Automation and Digital Monitoring: Modern chromatography systems are becoming smarter with automation and digital monitoring features. These advanced tools track the purification process in real time and can adjust performance automatically. If there’s an error or inconsistency, the system can detect and correct it instantly. This improves product quality and minimizes human error. Automated systems also help reduce labor costs and improve regulatory compliance. Companies can generate reports, log process data, and monitor equipment remotely. This shift toward smart manufacturing is making chromatography more reliable and efficient. As a result, digital solutions are becoming a standard part of chromatography setups.
- Environmental and Sustainability Considerations: Sustainability is now a major focus in chromatography system design. Companies are working to reduce their environmental footprint by cutting down on chemical use and waste. They’re using more eco-friendly solvents, recycling materials, and designing systems that require less water and energy. These efforts not only help the planet but also reduce operational costs. Governments and regulators are also encouraging green practices in pharmaceutical manufacturing. As a result, environmentally friendly chromatography solutions are gaining traction. More manufacturers are now prioritizing sustainable practices across all production stages from raw material handling to final drug purification.
- Customization for Niche Therapies: The rise of personalized medicine is changing how drugs are made. Treatments for rare diseases and specific patient groups often need smaller, customized batches. This creates a demand for flexible chromatography systems that can be tailored for unique purification needs. These systems must handle different molecule sizes and purity levels efficiently. Manufacturers are responding by developing modular, scalable systems. These allow quick adjustments for varying production sizes. As more niche therapies reach the market, the need for adaptable purification technologies will only grow. This trend is especially important in oncology, gene therapy, and other precision medicine fields.
Use Cases
- Biopharmaceutical Production: Preparative and process chromatography is essential in biopharmaceutical manufacturing. It helps purify complex biologic drugs such as insulin, monoclonal antibodies, and vaccines. These products must meet strict purity and safety standards before use in patients. Chromatography allows companies to remove unwanted substances like cell debris, DNA, and other impurities. It isolates the required proteins with high precision. This ensures the final drug product is effective and safe. Without chromatography, large-scale biologic production would be less reliable and more prone to contamination. As demand for biologic drugs grows, the use of chromatography systems continues to increase in production facilities worldwide.
- Vaccine Manufacturing: Vaccine production requires extremely pure ingredients. Preparative chromatography helps remove unwanted byproducts during this process. It separates active compounds from raw materials and impurities. This ensures the vaccine is safe, stable, and effective. Without proper purification, vaccines may lose potency or cause harmful reactions. Chromatography supports both traditional and modern vaccine platforms, including mRNA and viral vector-based vaccines. It also plays a role in quality control and process validation. As vaccine development speeds up globally, chromatography becomes even more important in ensuring public safety. Manufacturers rely on this technique to meet tight production timelines and regulatory standards.
- Gene and Cell Therapy: Gene and cell therapies are transforming how diseases are treated. These therapies involve complex biological materials like viral vectors or engineered cells. Preparative chromatography helps purify these critical components. For example, in CAR-T cell therapy, chromatography removes unwanted proteins and particles from the cell culture. This ensures that only the modified therapeutic cells are delivered to the patient. Similarly, in gene therapy, it helps isolate viral vectors carrying genetic material. The purification process is vital to avoid immune reactions or therapy failure. As advanced therapies gain momentum, chromatography plays a key role in ensuring product purity and patient safety.
- Enzyme Production: Enzymes are used in industries such as food processing, healthcare, and chemicals. Preparative chromatography helps produce enzymes that are pure and safe to use. It removes unwanted byproducts, microbial residues, and other contaminants. This process ensures the enzyme maintains its function and quality. For example, in the food industry, enzymes must meet safety standards before they’re added to products. In healthcare, enzymes used in therapies must be highly pure to avoid side effects. Chromatography ensures this level of quality. As enzyme use expands into new markets, the demand for reliable purification methods continues to rise.
- Food and Beverage Industry: Chromatography plays an important role in food and beverage manufacturing. It helps isolate proteins, nutrients, and natural flavors from complex mixtures. This ensures the final product has the right taste, nutrition, and safety. For example, it can be used to separate specific amino acids, vitamins, or flavor compounds. It also helps remove unwanted substances like allergens or toxins. In health-focused products, where ingredient purity is critical, chromatography becomes even more valuable. This technique supports product consistency and regulatory compliance. As consumers demand cleaner labels and better-quality foods, manufacturers turn to chromatography for precise ingredient control.
- Academic and Industrial R&D: Preparative chromatography is widely used in research labs and industrial R&D. It helps scientists separate and study biomolecules like proteins, DNA, or small chemical compounds. Researchers rely on it to purify samples before analyzing them. This supports discoveries in pharmaceuticals, biotechnology, materials science, and chemical engineering. In early-stage drug development, chromatography helps identify promising compounds. It also aids in testing the effects of different molecules. Academic institutions use it for fundamental research, while companies use it to develop new products. Its accuracy and reliability make chromatography a trusted tool in both educational and commercial research settings.
Conclusion
In conclusion, the preparative and process chromatography market is growing steadily, supported by rising demand for high-purity biologics and innovative therapies. Technologies like single-use systems, automation, and continuous manufacturing are transforming production efficiency and reliability. Pharmaceutical and biotech companies rely heavily on chromatography to meet strict quality and safety standards.
This trend is expected to continue as personalized medicine and biologic drugs expand. Regulatory support and strong research investment, especially in North America, will help drive further adoption. As a key tool in modern drug development and purification, chromatography systems are set to play an even bigger role in the future of healthcare manufacturing.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



