Table of Contents
Overview
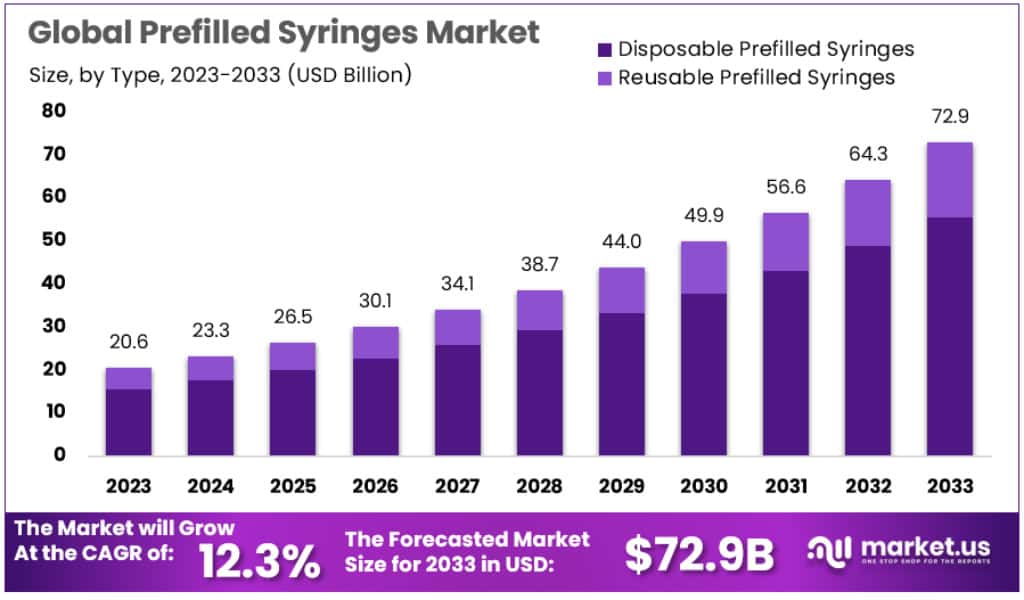
New York, NY – June 18, 2025: The Global Prefilled Syringes Market is projected to grow significantly, reaching approximately USD 72.9 billion by 2033 from USD 20.6 billion in 2023. This growth, at a compound annual growth rate (CAGR) of 12.3%, is supported by the rising prevalence of chronic and autoimmune conditions. Diseases such as diabetes, rheumatoid arthritis, and asthma require consistent drug administration. Prefilled syringes offer a reliable method for delivering essential therapies, including insulin and biologics listed in the WHO Essential Medicines List. These devices reduce dosing errors and improve patient compliance, making them essential in chronic disease management.
Regulatory bodies like the U.S. FDA and WHO have established guidelines for the development and quality control of prefilled syringes. The FDA classifies glass prefilled syringes as combination products, demanding rigorous standards for safety, biocompatibility, and performance. WHO’s technical standards for biologicals emphasize proper sealing, sterility, and manufacturing practices. These guidelines support industry innovation while ensuring patient safety. As a result, manufacturers are better equipped to develop compliant and globally accepted products, which facilitates broader market entry and acceptance.
The increasing adoption of biologics and biosimilars has further strengthened the role of prefilled syringes. Many injectable drugs under FDA’s Section 351 and 351(k) approvals are delivered through these systems. Prefilled syringes are well-suited to complex biologic therapies due to their accuracy and ease of use. In addition, advancements such as dual-chamber systems and lyophilized formulations improve drug stability and usability. These features help healthcare providers maintain consistent dosing and minimize contamination risks during preparation.
The push for patient-centric care has also driven demand for self-administered therapies. Prefilled syringes enable patients to manage their treatments at home, reducing dependence on healthcare facilities. Safety enhancements like needle guards and labeled instructions reduce the risk of misuse or injury. WHO and national health agencies support such models to lower the burden on healthcare systems and improve patient independence. These trends are especially relevant for long-term conditions requiring frequent injections outside hospital settings.
Prefilled syringes have become critical in pandemic preparedness and mass vaccination campaigns. WHO guidance on safe injection practices has encouraged their use during emergencies. Governments have responded by boosting fill–finish capabilities and reinforcing medical supply chains. Furthermore, international regulatory harmonization enables faster approvals and broader market access. Continuous quality monitoring, as mandated by the FDA’s post-licensing controls, strengthens market trust. These coordinated efforts ensure safety, scalability, and readiness for both routine care and public health emergencies.
Key Takeaways
- In 2023, the global prefilled syringes market reached a valuation of approximately USD 20.6 billion, driven by rising chronic disease cases.
- The market is anticipated to grow steadily at a compound annual growth rate (CAGR) of 12.3% between 2023 and 2033.
- By 2033, the prefilled syringes sector is expected to achieve a total value of nearly USD 72.9 billion worldwide.
- Disposable prefilled syringes dominated the market in 2023, accounting for over 76% of the total global market share.
- Reusable prefilled syringes represent a smaller segment but are preferred in cost-sensitive settings due to their multiple-use capability.
- Syringes used in diabetes care comprised over 51.9% of the market in 2023, indicating strong demand in chronic disease management.
- Although smaller in share, prefilled syringes for anaphylaxis are vital for emergency care in severe allergic reactions.
- Glass-based prefilled syringes held more than 52.7% market share in 2023, owing to their superior stability and chemical compatibility.
- Hospitals were the largest distribution channel for prefilled syringes, capturing over 48.9% market share in 2023.
- Europe emerged as the leading regional market in 2023, contributing 37.1% of global revenue with a market size of USD 7.6 billion.
Emerging Trends
- Auto-Disable and Safety-Engineered Design: The World Health Organization (WHO) recommends using auto-disable (AD) syringes in vaccination programs. These syringes come with needle-safety features that prevent reuse and reduce the risk of needlestick injuries. In the United States, both the Centers for Disease Control and Prevention (CDC) and the Occupational Safety and Health Administration (OSHA) require safety-engineered devices in hospitals and clinics. These devices help healthcare workers stay safe and prevent infections. The growing focus on infection control and worker safety is pushing the use of AD prefilled syringes in both low- and high-income countries.
- Single-Use, Manufacturer-Prefilled Packaging: The WHO prequalification program recognizes single-dose, manufacturer-prefilled syringes as ideal for vaccine delivery. These syringes reduce the chance of dosing errors and improve efficiency during mass immunization efforts. The CDC advises healthcare facilities to discard all prefilled syringes at the end of each clinic day. This helps avoid contamination and unused medicine waste. By using factory-filled units, clinics can streamline preparation and minimize human error. This trend supports better inventory control and more accurate dosing, especially in large-scale public health campaigns.
- Standardization and Technical Guidance: The U.S. Food and Drug Administration (FDA) supports international standards for prefilled syringes. ISO 11040-4 outlines safety requirements for glass prefilled syringes. The FDA also recommends extra testing to confirm safe connections between syringes, needles, and infusion ports. ISO 7886-1 and 7886-3 cover specifications for single-use syringes and prefilled AD variants. These standards ensure product compatibility and performance in clinical settings. Global regulatory alignment is driving safer and more consistent use of prefilled syringes across regions.
- Cold-Chain and Vaccine Integrity: Maintaining the cold chain is vital for prefilled vaccine syringes. WHO and CDC guidelines highlight the importance of temperature control during storage and transportation. Prefilled syringes, especially those made of glass, must be kept within recommended temperature ranges. Once removed from refrigeration, they should be used immediately to maintain drug stability and effectiveness. This practice is especially critical during immunization campaigns in remote or high-temperature regions. Adhering to cold-chain protocols ensures vaccines remain potent and safe for patients.
Use Cases
- Routine & Mass Vaccination Campaigns: The World Health Organization (WHO) uses compact prefilled auto-disable (CPAD) syringes in many immunization programs. These include vaccines like pentavalent and HPV. CPADs help improve vaccination accuracy and prevent reuse. They also reduce the need for cold chain handling and simplify logistics. These devices are particularly useful in low-resource settings. Their use ensures that each dose is safe, sterile, and easy to administer. They are now a preferred option in global Expanded Program on Immunization (EPI) efforts. The design eliminates manual dose preparation errors and reduces needle-stick injuries for health workers during mass campaigns.
- COVID‑19 & Influenza Immunization: The Centers for Disease Control and Prevention (CDC) recommends specific handling practices for prefilled syringes. For example, the Pfizer-BioNTech COVID-19 vaccine comes in prefilled glass syringes. After attaching a needle, the vaccine should be used immediately or within 4 hours. This ensures drug stability and patient safety. Prefilled syringes also reduce preparation time in clinical settings. They help in maintaining consistent dosing and limit the risk of contamination. These advantages have made them essential in pandemic and seasonal flu response. Their role is especially vital during large-scale immunization drives, where speed and accuracy are crucial.
- Clinical Settings & Injection Safety: Injection safety is a major concern in healthcare. The CDC’s Injection Safety Toolkit promotes safe syringe practices. It advises against reusing syringes or needles and strictly forbids accessing medication vials with the same syringe twice. This helps prevent the spread of infections such as hepatitis B, hepatitis C, and HIV. Prefilled syringes support this safety goal. They are designed for single use, which reduces handling errors. Their sealed nature also lowers the risk of environmental contamination. Hospitals and clinics increasingly rely on them to meet strict infection control protocols and safeguard patient health.
- Home & Consumer Use: Prefilled syringes are also used by patients at home. These include treatments for chronic conditions like diabetes and rheumatoid arthritis. The U.S. Food and Drug Administration (FDA) provides safety guidelines for home users. It advises proper disposal of sharps and warns against reusing any prefilled components. Most of these syringes are designed for one-time use only. This helps ensure correct dosing and prevents accidental infection. Their ease of use makes them suitable for elderly or non-professional users. With proper education and disposal tools, patients can manage treatment safely and independently at home.
- Device Quality Monitoring: The FDA continuously monitors the quality of injection devices. In 2023, it issued safety alerts about plastic syringes manufactured in China. These warnings did not apply to prefilled syringes. The FDA clarified that no safety risks have been reported for prefilled syringes at this time. This distinction reinforces confidence in prefilled formats. Their manufacturing undergoes strict regulatory checks. Prefilled syringes are also subject to stability and sterility testing. This monitoring protects patient safety and ensures product reliability. Healthcare providers trust these syringes because of their consistent performance and regulatory oversight.
Conclusion
In conclusion, prefilled syringes have become an important tool in modern healthcare. Their ease of use, safety features, and ability to deliver accurate doses make them suitable for hospitals, clinics, and even at-home care. These syringes help manage long-term conditions and support fast, safe vaccination during health emergencies. With strong support from global health organizations and clear safety guidelines, their use is expanding worldwide. Regulatory standards and innovation continue to improve product quality and reliability. As patient-centered care and biologic therapies increase, the demand for prefilled syringes is expected to grow. They play a key role in improving health outcomes across all care settings.


