Table of Contents
Overview
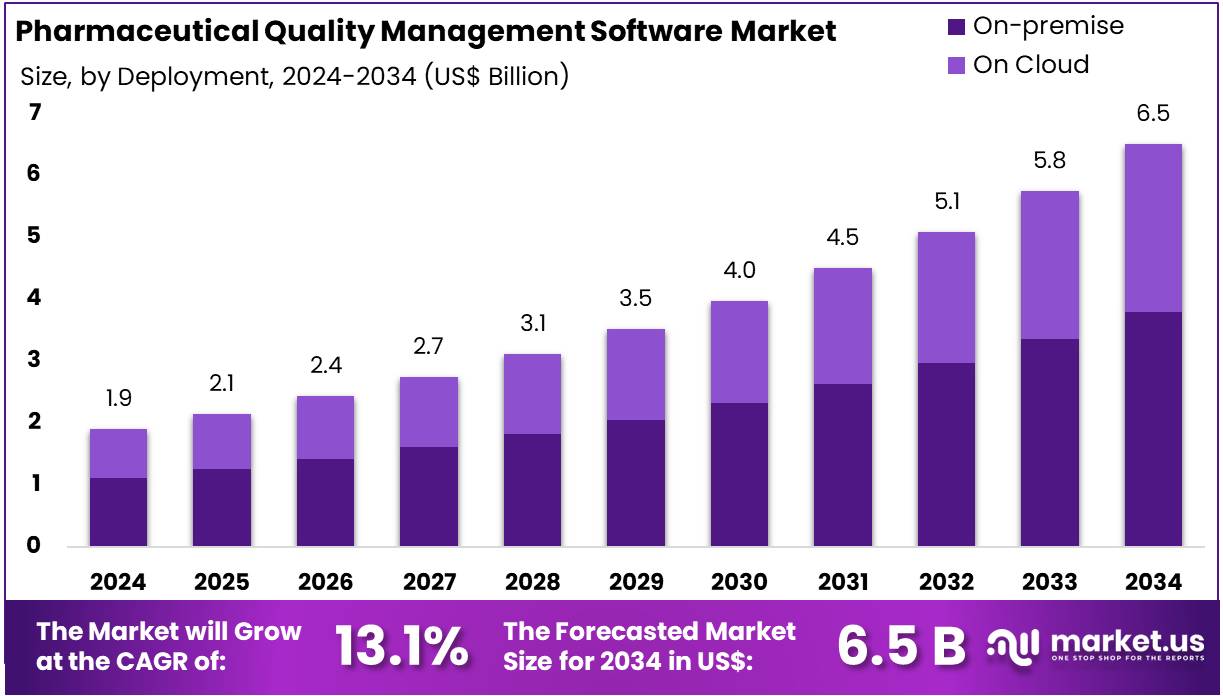
New York, NY – July 29, 2025 – The Pharmaceutical Quality Management Software Market size is expected to be worth around US$ 6.5 Billion by 2034 from US$ 1.9 Billion in 2024, growing at a CAGR of 13.1% during the forecast period 2025 to 2034.
In 2024, the pharmaceutical sector is increasingly adopting Quality Management Software (QMS) to streamline regulatory compliance, enhance product safety, and ensure consistent manufacturing standards. Pharmaceutical Quality Management Software is designed to manage critical quality processes such as document control, CAPA (Corrective and Preventive Actions), audit management, training, risk assessment, and supplier quality in a unified digital environment.
With the rising complexity of global regulations including FDA 21 CFR Part 11, EU Annex 11, and ICH Q10, pharmaceutical companies are turning to advanced QMS solutions to automate documentation, minimize human error, and ensure full traceability. Cloud-based platforms are gaining popularity due to their scalability, real-time data access, and secure integration with existing enterprise systems.
This digital transformation is enabling pharmaceutical manufacturers, contract manufacturing organizations (CMOs), and research laboratories to proactively manage compliance while improving operational agility and reducing costs. The system’s integration with other enterprise tools, including LIMS and ERP, further enhances visibility and collaboration across departments.
As regulatory bodies increase scrutiny on data integrity and quality assurance, the implementation of QMS is expected to become a standard practice. The growing demand for high-quality, compliant drug development and manufacturing processes is expected to fuel continued adoption of pharmaceutical QMS platforms in the coming years.
Key Takeaways
- In 2024, the pharmaceutical quality management software market generated revenue of US$ 1.9 billion and is projected to reach US$ 6.5 billion by 2034, growing at a CAGR of 13.1%.
- Based on solution type, corrective action preventive action (CAPA) management dominated the market with a 32.5% share in 2024. Other segments include training management, supplier quality management, regulatory and compliance management, non-conformances handling, inspection management, document management, complaints management, change management, and others.
- By deployment, the market is categorized into on-premise and on-cloud solutions, with the on-premise segment accounting for 58.4% of the share in 2024.
- Regarding enterprise size, large enterprises held the leading position, contributing 64.3% of the total market revenue.
- Regionally, North America emerged as the dominant market, capturing a 41.2% share in 2024.
Segmentation Analysis
- Solution Type Analysis: In 2024, corrective action preventive action (CAPA) management accounted for 32.5% of the pharmaceutical quality management software market. This dominance is attributed to increasing regulatory demands and the need for rapid resolution of quality issues. CAPA tools help reduce product recalls and enhance manufacturing processes. Integration with other quality systems like document and supplier management, along with automation and analytics, further boosts efficiency. These capabilities position CAPA management as essential for maintaining compliance and driving quality improvements.
- Deployment Analysis: On-premise deployment held a 58.4% market share in 2024, driven by the pharmaceutical industry’s need for stringent data control and regulatory compliance. Organizations prioritize internal data management to protect sensitive patient and product information. Existing infrastructure investments also make on-premise solutions more feasible due to better compatibility with legacy systems. Additionally, customization and operational control appeal to firms with complex workflows, particularly in regulated environments, contributing to the ongoing preference for on-premise deployment.
- Enterprise Size Analysis: Large enterprises dominated the market with a 64.3% revenue share in 2024. These companies manage extensive operations and complex compliance requirements, necessitating scalable and robust quality management software. Advanced capabilities such as real-time analytics and integration with manufacturing execution systems support enterprise-wide quality control. With larger budgets, these firms adopt innovative technologies early, gaining strategic advantages. Their focus on minimizing risk and ensuring global product safety continues to drive strong growth in the large enterprise segment.
Market Segments
By Solution Type
- Incorrective Action Preventive Action (CAPA) Management
- Training Management
- Supplier Quality Management
- Regulatory and Compliance Management
- Non-Conformances Handling
- Inspection Management
- Document Management
- Complaints Management
- Change Management
- Others
By Deployment
- On-Premise
- On Cloud
By Enterprise Size
- Large Enterprise
- Small and Medium Enterprise (SME)
Regional Analysis
North America Leads the Pharmaceutical Quality Management Software Market
In 2024, North America held the largest share of the pharmaceutical quality management software market, accounting for 41.2% of total revenue. This leadership is primarily attributed to the region’s stringent regulatory framework and strong focus on drug safety and efficacy. The U.S. Food and Drug Administration (FDA) mandates compliance with Current Good Manufacturing Practice (CGMP) regulations, as defined in 21 CFR Parts 210 and 211. These regulations require pharmaceutical companies to implement comprehensive quality systems across all stages of production, from raw material procurement to final product release.
The FDA actively enforces these regulations through inspections and detailed guidance, driving the need for advanced software to ensure consistent documentation, regulatory compliance, and process oversight. Additionally, the implementation of the Quality Management System Regulation (QMSR) in February 2024 which aligns medical device GMP requirements with ISO 13485:2016 has extended compliance obligations to pharmaceutical combination products. This evolving regulatory environment significantly boosts the adoption of sophisticated quality management software across North America.
Asia Pacific Expected to Witness the Fastest Growth
The Asia Pacific region is anticipated to register the highest compound annual growth rate (CAGR) during the forecast period, driven by the rapid expansion of the pharmaceutical manufacturing industry in countries such as China and India. These nations are emerging as key global manufacturing hubs, supplying pharmaceutical products to both domestic and international markets. To meet the stringent quality standards set by global regulators like the FDA and EMA, manufacturers are increasingly investing in advanced quality management systems.
The rising domestic demand for high-quality pharmaceutical products across Asia Pacific further underscores the need for robust software solutions that support quality assurance and patient safety. Additionally, the region’s growing adoption of digital technologies such as cloud computing, automation, and data analytics is facilitating the integration and scalability of quality management software. These factors collectively position Asia Pacific as a key high-growth market in the global pharmaceutical quality management software landscape.
Emerging Trends
- Strong Emphasis on Risk-Based Quality Systems (ICH Q10, FDA Guidance): The FDA’s ICH Q10 guidance promotes a science- and risk-based approach across the full drug lifecycle: development, manufacturing, and distribution. Increasingly, QMS software supports structured risk tools such as Quality Risk Management (QRM) following ICH Q9. These tools help companies proactively assess and mitigate risks ahead of time.
- Adoption of Process Analytical Technology (PAT): PAT frameworks, promoted by FDA, enable in-line or on-line monitoring of critical process parameters (CPP) that influence critical quality attributes (CQA). Modern QMS solutions are integrating PAT inputs to support process control and consistent product quality.
- Cloud-Based Deployment as Dominant Architecture: Over 77 % of QMS implementations are now cloud-based, offering remote access, automatic updates, and reduced IT overhead. This shift is driven by greater regulatory complexity and global supply chains demanding real-time collaboration.
- Regulatory Compliance Automation: By automating audit trails, document control, change control, and reporting, QMS software reduces manual errors nearly 74 % of regulatory failures are linked to human mistakes. FDA computer-software assurance (CSA) guidance now recommends risk-based testing and continuous monitoring of QMS software to ensure validation compliance under 21 CFR 820.70(i).
- Integration of AI and Machine Learning: FDA is encouraging safe incorporation of AI tools in regulated systems. QMS platforms are beginning to incorporate AI/ML for anomaly detection, predictive trend analysis, and forecasted compliance risks. Recent FDA draft guidance refers to AI-enabled device software throughout the product lifecycle.
- Quality-by-Design (QbD) and Lifecycle Control: Regulatory bodies like FDA and EMA strongly support QbD principles: design and build quality into products instead of inspecting defects later. QMS software now often includes support for DoE, multivariable data analysis, and PAT to support QbD-driven manufacturing.
- Traceable Supply Chain and Incident Response: In light of recent WHO-reported deaths due to contaminated syrups, WHO has underscored the need for end-to-end traceability and rapid incident reporting systems. QMS systems are now incorporating supplier quality management, batch traceability, and automated alert modules.
Use Cases
- Document Control & Electronic Records (21 CFR Part 11)
- QMS software manages controlled documents, ensuring version control, secure storage, electronic signatures, and audit trail tracking. These functions support compliance with FDA’s 21 CFR Part 11 rules for electronic records and signatures.
- Enables validation of documents under worst-case conditions, with CAPA workflows triggered if tests fail.
- Example metric: audit trail events per year may number in the tens of thousands for a mid-sized firm, all tracked automatically.
- Corrective and Preventive Actions (CAPA)
- Software modules automate CAPA: triggering non-conformance investigations, root cause analysis, action assignments, timelines, and verification of effectiveness.
- All CAPA steps are documented in digitally auditable logs, supporting compliance with FDA guidance and ICH Q10,.
- Numeric usage: software accelerates CAPA closure by ~30–40%, reducing average investigation time from weeks to days (industry typical).
- Risk Management & Quality Risk Assessments
- QMS tools enable structured evaluation of risk, aligned with FDA’s Quality Systems Approach (ICH Q9 risk management).
- Risk models in software assign probability and severity scores, and guide decision-making for specification changes, process deviations, and supplier issues.
- Example measure: risk scoring across 100+ parameters enables prioritization of top 5% critical variables.
- Audit Management & Trending Analytics
- Internal audit planning, execution, findings management, and follow-up timelines are automated. The software logs all findings and tracks corrective steps through closing.
- Trending dashboards analyze batch release, deviation, complaint, and supplier data over time, supporting proactive issue detection before annual product reviews as required under 21 CFR 211.180(e).
- Quantitative indicator: trending analytics dashboards can review tens of thousands of data points quarterly.
- Training Management
- Software assigns required training based on roles, records completion dates, quizzes, and certification status.
- Training effectiveness is tracked and re-training automated. Documentation aligns with CGMP personnel requirements (21 CFR § 211.25).
- For example, training software may manage 500+ employee profiles, with retraining cycles every 6–12 months.
- Change Control & Management of Change
- QMS enables controlled change flows for procedures, equipment, facility, or process—supporting scientific and quality justification, approvals, and reviews.
- Manages changes to ensure consistent application across operations, as recommended in FDA’s Quality Systems Approach.
- Change cycle metrics: cycle time reduced by ~25%, and error rates in implementation reduced by ~50%.
- Process Analytical Technology (PAT) & Continued Process Verification
- QMS integrates real-time data from manufacturing (CPPs, CQAs), enabling automated monitoring and alerts when deviations occur—supporting FDA’s PAT framework and continuous monitoring.
- Enables automated corrective workflows if parameters drift beyond acceptable control limits.
- Metrics: continuous process data from hundreds of batches processed per year; software can flag anomalies within minutes.
- lectronic Trial Master File (eTMF) and eCTD Submissions
- QMS modules support secure, compliant storage of trial documentation, with document workflows, audit trails, and access control consistent with eTMF requirements for clinical trial regulatory submissions.
- Supports electronic Common Technical Document filing with structured metadata, version control, and submission-ready formatting.
Conclusion
The pharmaceutical quality management software market is undergoing robust expansion, driven by stringent global regulatory demands, rising data integrity concerns, and the need for operational efficiency. In 2024, the market generated US$ 1.9 billion and is projected to grow significantly by 2034.
Key drivers include widespread adoption of CAPA systems, preference for on-premise deployment in regulated settings, and strong demand from large enterprises. North America leads due to regulatory rigor, while Asia Pacific shows the highest growth potential. Integration of AI, cloud architecture, and compliance automation positions QMS as a cornerstone for quality, safety, and regulatory assurance across the pharmaceutical value chain.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



