Table of Contents
Overview
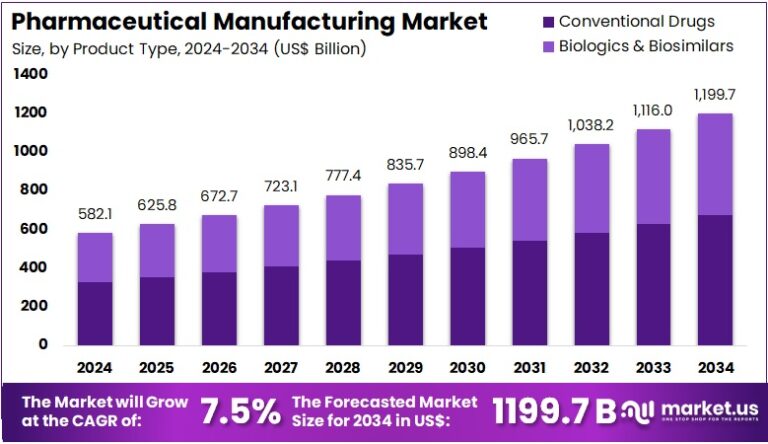
New York, NY – July 31, 2025 : The Pharmaceutical Manufacturing Market is projected to grow from US$ 582.1 billion in 2024 to US$ 1,199.7 billion by 2034. This growth reflects a CAGR of 7.5% during the forecast period of 2025 to 2034. Rising global demand for accessible and effective healthcare solutions is a major factor behind this trend. With healthcare systems under pressure to deliver value-based care, the pharmaceutical industry is responding with innovations that enhance efficiency and lower production costs while maintaining product quality and regulatory compliance.
One of the key growth drivers is the push for more efficient and faster drug production methods. Pharmaceutical manufacturers are now adopting advanced technologies such as automation and continuous manufacturing. These innovations help to reduce operational costs and improve scalability. Companies are also investing in process optimization to ensure timely delivery and minimize supply chain disruptions. The shift toward leaner manufacturing practices supports the industry’s need to meet growing patient demands without compromising safety or efficacy.
Another major trend is the increasing focus on biologics and biosimilars. These therapies are gaining popularity due to their effectiveness in treating chronic conditions such as cancer, diabetes, and autoimmune disorders. As patient populations grow and age, the demand for specialized treatments continues to rise. Biologics offer targeted solutions, but their production is complex and expensive. This complexity is pushing pharmaceutical companies to refine their manufacturing capabilities to ensure higher output, greater affordability, and broader access to critical medicines.
Strategic partnerships are playing a vital role in advancing manufacturing capabilities. In June 2023, Pfizer and Samsung Biologics entered into a collaboration to expand biosimilar production. This partnership focuses on therapies in immunology, oncology, and inflammation. It reflects the industry’s commitment to increasing global supply and lowering costs for biologic drugs. Through joint ventures like this, pharmaceutical companies are addressing the challenge of rising healthcare expenditures while ensuring patients receive high-quality, life-saving treatments on time and at scale.
The pharmaceutical manufacturing landscape is undergoing a transformative shift. Innovations in production methods, such as real-time monitoring, AI integration, and modular facilities, are redefining efficiency standards. Companies that embrace these technologies will gain a competitive advantage in meeting regulatory requirements and market demands. With chronic diseases on the rise and global access to medicines becoming more critical, the industry must continue to evolve. This evolution presents a significant opportunity for stakeholders to drive both economic growth and improved healthcare outcomes worldwide.
Key Takeaways
- In 2024, the global pharmaceutical manufacturing market generated $582.1 billion and is projected to reach $1,199.7 billion by 2034 at 7.5% CAGR.
- Conventional drugs led the product type segment in 2024, capturing a significant 56.2% market share over biologics and biosimilars.
- Outsourced drug development dominated with a 61.5% market share, reflecting growing reliance on external manufacturing partners for efficiency and scalability.
- Tablets were the top formulation in 2024, accounting for the largest revenue share of 45.3% across all pharmaceutical dosage forms.
- Oral route of administration was the market leader, holding a 57.5% revenue share due to its convenience and patient compliance.
- Cardiovascular diseases drove the therapy area segment, commanding a dominant 47.3% share in pharmaceutical manufacturing applications.
- Adults represented the largest age-based consumer group, with a 54.5% market share, highlighting their higher medication consumption rates.
- North America led the regional market in 2024, securing a 42.4% share thanks to robust infrastructure and advanced healthcare systems.
Regional Analysis
North America led the pharmaceutical manufacturing market in 2024, holding a 42.4% revenue share. This dominance is driven by regulatory approvals and strong government support. The FDA approved 55 novel drugs in 2023, a 15% rise from 2022. US pharmaceutical facilities expanded capacity by 12%, while exports increased by 9%. Canada saw a 22% rise in patented medicine production. The CHIPS and Science Act allocated $2 billion to manufacturing technologies. Biologic drug production in the US grew by 18%, boosting North America’s leadership in this sector.
The Asia Pacific region is expected to grow at the highest CAGR during the forecast period. This growth is fueled by rising drug approvals and manufacturing support. China approved 42% more new drugs in 2023, while India’s output rose by 17% due to trade incentives. Japan and South Korea also reported strong increases in facility registrations and biologic approvals. Australia expects a 30% rise in site approvals in 2024. Government investments, including India’s $3.2 billion funding, support Asia Pacific’s growth in advanced pharmaceutical manufacturing.
Segmentation Analysis
Product Type Analysis
In 2024, the conventional drugs segment dominated with a 56.2% market share. These chemical-based drugs remain popular due to their affordability and proven efficacy. Healthcare providers continue to favor conventional drugs to manage treatment costs effectively. The growing prevalence of chronic conditions like diabetes and hypertension further boosts demand. With established production infrastructure and the ability to treat a wide range of diseases, conventional drugs are expected to maintain strong market presence. Cost control and broad applicability will drive sustained adoption globally.
Drug Development Analysis
Outsourcing held a dominant 61.5% market share in 2024, reflecting a shift in development strategy. Pharmaceutical companies increasingly rely on external partners for cost reduction and faster time-to-market. Contract research and manufacturing organizations offer specialized expertise. With drug development growing more complex, outsourcing enhances flexibility and efficiency. As the demand for innovative therapies rises, this trend is expected to continue. Outsourcing clinical trials, R&D, and production helps companies scale operations while focusing internal resources on core competencies and regulatory compliance.
Formulation Analysis
Tablets accounted for 45.3% of revenue in 2024, leading the formulation segment. Their popularity stems from ease of use, cost-efficiency, and long shelf-life. Tablets are widely used for chronic conditions like hypertension and diabetes. Ongoing innovations in tablet formulations, such as controlled-release technology, enhance therapeutic outcomes. These improvements also promote better patient adherence. As chronic disease rates grow worldwide, the demand for reliable oral dosage forms like tablets will increase. Manufacturers benefit from established infrastructure and production scalability in this segment.
Route of Administration Analysis
The oral route dominated administration methods in 2024, generating 57.5% of market revenue. Patients prefer oral medications due to ease of use and non-invasiveness. This method supports adherence, especially for chronic disease treatment. Oral drugs are ideal for long-term regimens, making them popular in managing conditions like diabetes and heart disease. Innovations in oral formulations, such as improved bioavailability and slow-release mechanisms, continue to enhance this route. These advancements contribute to strong patient compliance and make oral drugs the preferred delivery method.
Therapy Area Analysis
Cardiovascular diseases held a significant 47.3% market share in 2024. The rising global burden of heart-related conditions is a major driver. An aging population and lifestyle changes increase the need for cardiovascular medications. Pharmaceutical innovations target specific conditions like hypertension and heart failure. Preventative treatments and better disease management are also gaining attention. With enhanced formulations and broader access to care, demand for cardiovascular therapies is expected to grow. This segment will remain a key focus for pharmaceutical companies and healthcare systems worldwide.
Age Group Analysis
Adults represented 54.5% of the market in 2024, driven by the rising adult population. Lifestyle-related conditions such as obesity, diabetes, and hypertension are more common in adults. These health concerns increase the need for pharmaceutical interventions. Adults also form the primary group requiring long-term medication use. As the global adult population ages, their healthcare needs are growing. This demographic will continue to demand chronic disease treatments. Pharmaceutical companies are expected to focus on developing and marketing products tailored to this expanding segment.
Key Market Segments
By Product Type
- Biologics & Biosimilars
- Monoclonal Antibodies
- Vaccines
- Cell & Gene Therapy
- Others
- Conventional Drugs
By Drug Development
- In-house
- Outsource
By Formulation
- Tablets
- Suspensions
- Sprays
- Powders
- Injectable
- Capsules
- Others
By Route of Administration
- Oral
- Topical
- Parenteral
- Inhalations
- Others
By Therapy Area
- Cardiovascular Diseases
- Respiratory Diseases
- Pain
- Diabetes
- Cancer
- Other
By Age Group
- Children & Adolescents
- Adults
- Geriatric
Key Players Analysis
Key players in the pharmaceutical manufacturing market are focusing on innovation, cost control, and capacity expansion. They are investing in technologies like continuous manufacturing, automation, and 3D printing. These advancements help boost production efficiency and lower operational costs. Companies also maintain strict regulatory compliance to meet global quality standards. Strategic collaborations with research institutions, contract manufacturers, and healthcare providers are common. These partnerships help extend market reach. Additionally, growing demand in emerging markets is pushing manufacturers to offer affordable, accessible pharmaceutical solutions tailored to local healthcare needs.
Pfizer Inc., based in New York City, stands out as a global biopharmaceutical leader. The company develops, produces, and distributes medicines and vaccines for therapeutic areas like oncology, cardiology, and immunology. Pfizer focuses on research and innovation by investing in advanced manufacturing technologies. This enhances efficiency and quality. The company also builds strong alliances with healthcare organizations. With a solid global footprint and manufacturing network, Pfizer leads in delivering pharmaceuticals across both developed and emerging markets. Its strategic approach ensures continued growth and market leadership.
Top Key Players in the Pharmaceutical Manufacturing Market
- Sanofi SA
- Samsung Biologics
- Pfizer, Inc
- Novartis AG
- MilliporeSigma
- Johnson & Johnson
- Eli Lilly and Company
- AstraZeneca
Emerging Trends
1. Adoption of Advanced Manufacturing Technologies
Pharmaceutical companies are shifting from traditional methods to advanced manufacturing technologies. Tools like automation, robotics, and continuous manufacturing are now widely used. These innovations help increase production speed and reduce errors. Automation also improves accuracy and consistency across batches. Robotics supports safer handling of materials and precise operations. Continuous manufacturing allows faster drug production compared to batch processes. This shift helps companies respond quicker to market demands. Overall, these modern methods offer better efficiency and higher quality, making them essential in today’s fast-paced pharmaceutical environment.
2. Growing Focus on Personalized Medicine
There’s a rising demand for personalized treatments in healthcare. Pharmaceutical companies are now creating drugs tailored to individual patients or small groups. This approach requires flexible and precise manufacturing systems. Traditional mass production doesn’t work well for personalized medicine. Each batch may need different ingredients, doses, or delivery methods. This change calls for adaptive technologies and customized production lines. Personalized medicine also improves treatment results by targeting a person’s unique biology. As a result, the entire manufacturing process must be more agile and patient-specific.
3. Increased Use of Artificial Intelligence (AI)
Artificial Intelligence (AI) is becoming a key part of drug manufacturing. AI helps predict outcomes during production and reduces the risk of human error. It can also detect quality issues early, before they affect large batches. AI-powered systems monitor equipment performance and adjust processes in real-time. This means fewer delays and better product quality. In drug development, AI speeds up research and testing by analyzing large amounts of data quickly. By using AI, pharmaceutical companies can improve efficiency, cut costs, and produce safer, more effective drugs.
4. Sustainable and Eco-Friendly Production
Sustainability is now a major goal in pharmaceutical manufacturing. Companies are working to lower waste, reduce energy use, and follow eco-friendly practices. This shift is driven by climate concerns and tougher regulations on pollution. Manufacturers are investing in cleaner technologies, recyclable materials, and more efficient processes. They also focus on reducing their carbon footprint and water consumption. Green production methods not only help the planet but also improve brand image. Consumers and regulators both expect the industry to operate responsibly. Sustainable practices are now a must-have in this evolving market.
5. Outsourcing and Contract Manufacturing
Pharmaceutical firms are increasingly outsourcing parts of their manufacturing process. They often work with contract manufacturing organizations (CMOs) to produce drugs more efficiently. Outsourcing helps companies reduce costs and focus on research and innovation. CMOs have specialized equipment and expertise, allowing for high-quality production. This also speeds up the time it takes to bring new drugs to market. Small and mid-sized firms especially benefit, as they may lack large-scale manufacturing facilities. As demand grows, the role of outsourcing in pharmaceutical manufacturing is becoming more strategic and essential.
6. Stronger Focus on Supply Chain Resilience
Recent global disruptions have exposed weaknesses in pharmaceutical supply chains. In response, manufacturers are strengthening their supply networks. Many companies are now producing drugs closer to the markets they serve. This helps reduce delays and transportation risks. Digital tools are also being used to track materials, manage inventory, and predict supply issues. A resilient supply chain ensures steady drug availability, even during emergencies. By improving visibility and flexibility, companies can better handle future disruptions. Building a reliable supply chain is now a top priority for the pharmaceutical industry.
Use Cases
1. Fast-Tracking Vaccine Production
Modern manufacturing methods help speed up vaccine production, especially during health emergencies. Technologies like continuous manufacturing and automation allow companies to produce vaccines quickly and at scale. These systems reduce the time needed for manual handling and increase efficiency. With automation, manufacturers can create millions of doses in a short time. This was especially important during the COVID-19 pandemic. The ability to rapidly respond to global health crises depends on these advanced systems. They make it possible to go from lab to large-scale supply faster than ever before, helping save lives when every second counts.
2. Producing Generic Drugs Efficiently
Generic drugs must be affordable, high-quality, and made in large quantities. To meet this goal, manufacturers use automated production lines. Automation helps reduce labor costs and human error. It also improves consistency and speeds up the manufacturing cycle. These systems are ideal for producing large volumes without increasing expenses. By using robotics and smart machinery, companies can ensure that every tablet or capsule meets strict safety standards. This helps make essential medicines accessible to more people around the world. Efficient production also supports healthcare systems by keeping drug prices low.
3. Scaling Up for Global Demand
After a drug receives approval, global demand can grow fast. Pharmaceutical companies use modular production units to respond quickly. These units can be added or expanded without stopping production. This flexibility allows manufacturers to scale up output when demand increases. It’s especially useful for vaccines, chronic disease treatments, or seasonal drugs. Modular systems save time and cost compared to building new facilities. They help companies adjust production levels based on real-time needs. As a result, patients across different countries can get access to essential drugs without delay.
4. Real-Time Quality Control
Modern factories use smart sensors and AI to monitor quality in real-time. These technologies check drug composition, temperature, and other factors during production. If anything goes wrong, the system alerts staff immediately. This helps prevent faulty products from reaching the market. Real-time monitoring also reduces the need for post-production testing. It saves time and ensures every batch meets safety standards before it leaves the facility. With automated checks, companies can keep up with strict regulations while maintaining high output. It’s a smarter, safer way to ensure drug quality.
5. Flexible Facilities for Personalized Medicine
Personalized medicine is becoming more common. It requires small-batch production tailored to individual patients. To meet this need, companies build flexible manufacturing units. These facilities can quickly switch between different drugs or formulations. They use modular systems and digital controls to manage changeovers. This flexibility is ideal for rare diseases or customized treatments. It also reduces downtime between batches. As personalized therapies grow in demand, having adaptable production spaces becomes crucial. It allows manufacturers to meet unique patient needs while staying efficient and cost-effective.
6. Remote Monitoring and Maintenance
Modern pharmaceutical plants use connected machines that can be monitored remotely. Technicians can track equipment status in real time and even perform maintenance from another location. This reduces downtime and improves overall efficiency. It’s especially helpful in remote areas or during travel restrictions. If a machine has a problem, alerts are sent instantly. Remote diagnostics can solve issues faster than on-site visits. This kind of system ensures continuous operation and better resource planning. It also enhances safety by limiting the need for frequent in-person checks.
7. Reducing Waste Through Continuous Manufacturing
Traditional batch processing often leads to waste and inefficiency. Continuous manufacturing offers a better solution. In this method, materials are fed into the system continuously, not in separate batches. This reduces leftover materials and energy use. It also improves product consistency and shortens production times. With fewer interruptions, the process becomes more efficient and eco-friendly. Companies can produce the same amount of medicine using fewer resources. Continuous manufacturing supports both cost savings and sustainability goals. It’s becoming a preferred method for many pharmaceutical producers worldwide.
Conclusion
In conclusion, the pharmaceutical manufacturing market is evolving rapidly with a strong focus on innovation, efficiency, and global accessibility. Companies are adopting advanced technologies like automation, AI, and continuous manufacturing to boost productivity and meet rising healthcare demands. The shift toward biologics, personalized medicine, and eco-friendly practices is also reshaping production strategies. Outsourcing and strategic partnerships are helping firms stay competitive while ensuring quality and scalability. As chronic diseases grow and healthcare needs rise worldwide, the market will continue to expand. This dynamic environment offers strong opportunities for manufacturers who embrace change and invest in flexible, high-quality production systems to deliver better outcomes for patients.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



