Introduction
The Pharmaceutical-Grade Lactose Market Size is projected to grow significantly, reaching approximately USD 3.4 billion by 2033 from its 2023 value of USD 2 billion. This growth reflects a compound annual growth rate (CAGR) of 5.7% between 2024 and 2033. Pharmaceutical-grade lactose is a critical ingredient in tablets and capsules, driven by rising global demand for medicines and advanced drug delivery methods.
The increasing need for medications worldwide is boosting demand for excipients like pharmaceutical-grade lactose. As the global population ages and chronic diseases rise, the pharmaceutical industry is scaling up production. This growth directly impacts the need for lactose, which ensures the safe and effective formulation of drugs. Additionally, regulatory evaluations have deemed the BSE risk in pharmaceutical-grade lactose negligible, further strengthening consumer confidence in its safety.
Advancements in drug delivery methods, including dry powder inhalation systems, are creating new opportunities for lactose applications. Companies like DFE Pharma are developing specialized lactose grades to meet the unique requirements of these innovative systems. These advancements enhance treatment options and expand the market potential for lactose excipients.
Strategic industry partnerships are also shaping market dynamics. For example, Kerry Bio-Science and Foremost Farms USA formed an alliance to enhance their market presence and product offerings. Such collaborations drive innovation and extend market reach, ensuring the pharmaceutical-grade lactose sector remains competitive.
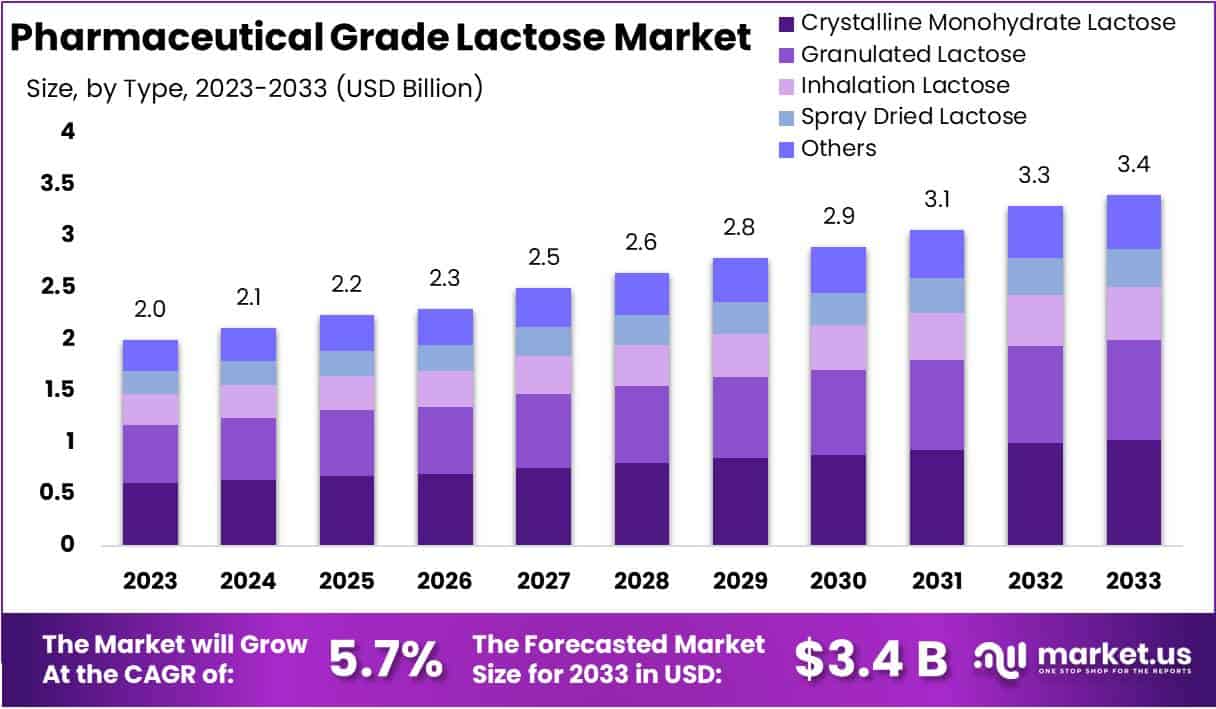
Key Takeaways
- The Pharmaceutical Grade Lactose Market is forecasted to hit USD 3.4 billion by 2033, growing steadily at a 5.7% CAGR from 2024 to 2033.
- Cost-effectiveness drives demand, with anhydrous lactose gaining popularity in direct compression processes, significantly contributing to market expansion.
- Reactive impurities in lactose, intolerance issues, and limited compatibility with some drugs present significant challenges to market growth.
- Advancements in the pharmaceutical industry are creating new growth opportunities, spurring increased demand for pharmaceutical-grade lactose globally.
- The use of anhydrous lactose in direct compression processes is a prevailing trend, simplifying production and enhancing efficiency in pharmaceutical manufacturing.
- Crystalline Monohydrate Lactose held a dominant 30.3% market share in 2023, making it a key segment in the market.
- Capsule manufacturing accounted for 72.1% of the application market share in 2023, highlighting its strong position in the pharmaceutical lactose market.
- North America led the market in 2023 with a 45.3% share and USD 0.89 billion market value, showcasing regional dominance.
- Asia-Pacific is witnessing rapid growth, driven by an expanding pharmaceutical industry and increasing healthcare investments across the region.
- Rising lactose intolerance, drug formulation technology advancements, and the emergence of personalized medicine are shaping global market trends.
Regional Analysis
In 2023, North America held a dominant market position with a 45.3% share, valued at USD 0.89 Billion. This leadership is driven by advanced healthcare infrastructure and substantial investments in pharmaceutical R&D. The region benefits from stringent regulatory frameworks that ensure high-quality standards. Additionally, major pharmaceutical companies and rising demand for drug formulations contribute to North America’s continued prominence in the pharmaceutical-grade lactose market.
Europe closely follows North America, benefiting from a dynamic pharmaceutical sector and extensive research initiatives. Government policies that encourage healthcare advancements also play a significant role. Furthermore, the region is witnessing an increase in innovative drug delivery systems. The growing elderly population, requiring safe excipients such as pharmaceutical-grade lactose, further fuels market growth in Europe, making it a key player in this space.
The Asia-Pacific region is experiencing rapid growth, driven by the expanding pharmaceutical industries in countries like China and India. Rising healthcare investments and growing awareness of generic medications are major contributors to this trend. Improved regulatory scenarios have also helped create a favorable environment. Additionally, the cost-efficient manufacturing landscapes in these countries attract global players, helping drive the demand for pharmaceutical-grade lactose and expanding the market in the region.
Latin America and the Middle East/Africa are emerging markets showing considerable potential. Healthcare sector expansions, greater access to medicines, and rising healthcare expenditures are driving growth in these regions. As they continue to develop their pharmaceutical and healthcare infrastructures, demand for high-quality pharmaceutical ingredients like lactose is expected to surge. The increasing prevalence of lactose intolerance and advances in drug formulation technologies will continue to shape the market dynamics across these regions.
Emerging Trends
- Growing Demand in Emerging Markets: The demand for pharmaceutical-grade lactose is rising in countries like India and Brazil. These regions are expanding their pharmaceutical manufacturing sectors due to increased investments in healthcare. A significant driver of this growth is the production of generic medications. As these countries focus on boosting their pharmaceutical output, the need for high-quality lactose used in drug formulations is steadily increasing. This trend is expected to continue as healthcare infrastructure in emerging markets improves and local pharmaceutical companies scale up operations to meet growing demands.
- Advancements in Lactose Formulations: Innovations in lactose processing are transforming its applications in pharmaceuticals. New grades, including spray-dried and granulated lactose, are now widely available. These enhanced forms improve the functionality of lactose in drug formulations. They also optimize the tablet manufacturing process and enhance drug delivery efficiency. By adopting these advanced formulations, pharmaceutical companies can create higher-quality products that meet both regulatory requirements and consumer expectations. The focus on formulation advancements is driving continuous improvements in lactose-based drug manufacturing.
- Emphasis on Quality and Purity: Meeting strict regulatory standards is a growing priority for pharmaceutical-grade lactose manufacturers. There is a strong focus on ensuring high levels of quality and purity. To achieve this, companies are using advanced purification techniques. These methods help remove impurities and contaminants, ensuring the lactose meets pharmaceutical-grade specifications. Such efforts are critical in maintaining compliance with international regulations and ensuring the safety and efficacy of drugs. The trend highlights the industry’s commitment to producing premium-quality lactose for pharmaceutical use.
Use Cases
- Tablet Manufacturing: Pharmaceutical-grade lactose is commonly used in making tablets. It acts as a filler and binder, giving the tablets bulk and ensuring even distribution of active ingredients. Around 60%–70% of pharmaceutical dosage forms contain lactose. This highlights its importance in tablet production. Lactose also improves the stability and consistency of tablets, making it a preferred choice for manufacturers. Its compatibility with various active ingredients further adds to its wide usage in tablet formulations.
- Capsule Manufacturing: Lactose plays a vital role in capsule formulations as a diluent. It ensures accurate dosing of active pharmaceutical ingredients. Its excellent flow properties make capsule filling processes efficient and precise. Manufacturers prefer lactose for its consistent particle size and minimal interaction with other ingredients. These properties ensure the capsules meet quality standards. Additionally, lactose enhances the stability and bioavailability of the active ingredients in capsules.
- Inhalation Products: Specialized lactose grades, such as inhalation lactose, are essential in dry powder inhalers. They serve as carriers for active drugs in respiratory therapies. Lactose helps deliver the medication effectively to the lungs. Its fine particle size and uniform distribution ensure optimal performance of the inhalers. The use of lactose in inhalation products supports treatments for asthma, chronic obstructive pulmonary disease, and other respiratory conditions.
- Biologic Drug Formulations: Lactose is critical in stabilizing protein-based drugs used in biologics. It maintains the drug’s efficacy and extends their shelf life. Biopharmaceuticals increasingly rely on lactose as an excipient due to its compatibility and protective properties. This role has grown in importance with the rise of biologic drugs. Lactose also helps maintain the structural integrity of proteins, ensuring reliable therapeutic effects for patients.
Conclusion
In conclusion, the pharmaceutical-grade lactose market is poised for steady growth, driven by rising global demand for medicines, aging populations, and chronic disease management. Innovations in drug delivery systems, like dry powder inhalers and advancements in lactose formulations further open up new opportunities. North America currently leads the market, followed closely by Europe and rapidly expanding regions such as Asia-Pacific. While challenges like lactose intolerance and compatibility with certain drugs exist, ongoing developments in quality control and new product formulations are addressing these issues. As the pharmaceutical industry continues to evolve, the demand for high-quality excipients like pharmaceutical-grade lactose is expected to remain robust.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



