Table of Contents
Introduction
The global pharmaceutical excipients market is on track for robust growth, expected to expand from USD 6.6 billion in 2023 to USD 11.8 billion by 2032, at a compound annual growth rate (CAGR) of 5.8%. This surge is driven by innovation in drug formulation, where novel excipients play a critical role in enhancing drug delivery, stability, and effectiveness. Modern manufacturing processes, including continuous production, are being adopted to advance the quality and application of these excipients, showcasing significant industry progress.
Stringent quality control and safety regulations have heightened within the sector, compelling pharmaceutical companies to uphold rigorous standards. New guidelines and standards are being developed for testing and verifying the purity and safety of excipients. Programs such as the USP’s Ingredient Verification Program are pivotal in ensuring high-level quality assurance, reflecting an industry-wide emphasis on safeguarding excipient integrity.
Advancements in supply chain management are vital due to excipients’ crucial role in drug safety and efficacy. Recent strategies are centered on enhancing traceability and understanding the source of excipients to mitigate risks associated with contamination or supply disruptions. Efforts to harmonize international standards are strengthening, fostering global cooperation and ensuring a resilient supply chain across the pharmaceutical industry.
The regulatory landscape is evolving, with a move towards more proactive frameworks to manage the complexities associated with novel excipients. This shift involves collaborative efforts among formulators, R&D scientists, and regulatory bodies, aiming to develop standards that cater to current market needs while anticipating future challenges. Such engagement is essential for fostering innovation and ensuring the safe incorporation of excipients into pharmaceutical products.
Recent developments underline the industry’s dynamic nature. In September 2024, Evonik launched a new spray drying facility in Germany, enhancing the supply of oral excipients. Lubrizol’s plan to build a significant manufacturing site in India by July 2024 highlights efforts to meet regional demands. Additionally, collaborations like that between BASF Pharma Solutions and IFF Pharma Solutions in November 2023 are set to revolutionize digital pharmaceutical formulation, showcasing the sector’s forward-thinking approach to embracing technological advancements.
Key Takeaways
- The pharmaceutical excipients market is projected to grow from USD 6.6 billion in 2022 to about USD 11.8 billion by 2032.
- This market is expected to see a 5.8% compound annual growth rate (CAGR) between 2022 and 2032, indicating significant expansion potential.
- Organic chemicals led the product types in 2022, favored for their non-toxic attributes and effectiveness.
- Oral formulations dominated the 2022 market due to their extensive benefits and disease control efficacy.
- Fillers and diluents were the most prevalent functionality types, primarily used in creating tablets and capsules.
- In 2022, Europe held the largest revenue share in this market, over 38%.
- The Asia-Pacific region is anticipated to experience the fastest growth compared to other areas covered in the report.
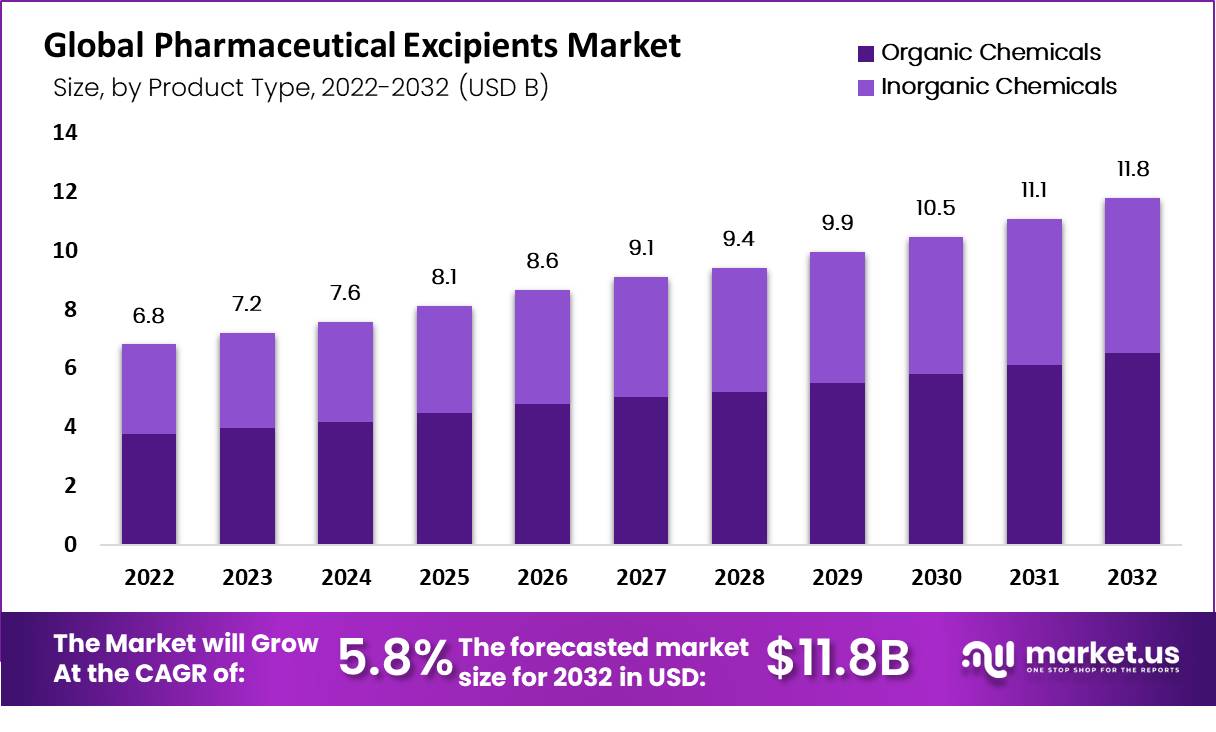
Pharmaceutical Excipients Statistics
- The total market size in 2022 was $6.8 billion.
- In 2023, the market size is projected to be $7.2 billion.
- The market size is expected to reach $7.6 billion in 2024.
- By 2025, the market size will grow to $8.1 billion.
- The projected market size for 2026 is $8.6 billion.
- In 2027, the market is anticipated to be $9.1 billion.
- The market size will increase to $9.4 billion in 2028.
- By 2029, it is expected to grow to $9.9 billion.
- The market size is forecasted to be $10.5 billion in 2030.
- In 2031, the market size is projected to reach $11.1 billion.
- The market is expected to grow to $11.8 billion by 2032.
- The market is forecasted to grow at a CAGR of 5.8% from 2022 to 2032.
Emerging Trends
- Coprocessed Excipients: The pharmaceutical industry is increasingly adopting coprocessed excipients, which combine multiple functionalities into a single component. This integration simplifies the production process by reducing the variety of excipients needed, thus improving manufacturing efficiency. Such advancements are particularly beneficial in continuous manufacturing settings where consistency in performance is essential. Coprocessed excipients streamline operations and potentially reduce costs, making them a key trend in modern pharmaceutical manufacturing.
- Nanotechnology in Excipients: The use of nanotechnology in excipients is growing, especially in the area of tablet coatings. Nanocoatings help control the release rates of drugs, thereby enhancing the bioavailability of active pharmaceutical ingredients (APIs). This technology also facilitates the inclusion of functional materials like polymers and antimicrobial agents directly on the tablet’s surface. The ability to finely tune drug release and add protective features through nanotechnology is a significant trend that could revolutionize tablet manufacturing.
- Advanced Manufacturing Techniques: Innovations in 3D printing and digital manufacturing are transforming the use of excipients. These advanced techniques allow for the production of highly personalized medications that cater to individual patient needs more precisely. Additionally, they support decentralized manufacturing, enabling quicker adaptations to meet regional health demands. The trend towards personalization and flexibility in drug production through advanced manufacturing is reshaping the pharmaceutical landscape.
- Regulatory Innovations: There is a notable shift towards developing regulatory frameworks for novel excipients. This movement aims to facilitate the introduction of innovative drug formulations by ensuring the safety and efficacy of new excipients. Regulatory bodies are exploring new methods to assess these components faster, which could accelerate the availability of groundbreaking drugs in the market. The trend towards regulatory innovation supports the faster integration of advanced excipients, fostering further advancements in pharmaceuticals.
Use Cases
- Generic Drug Manufacturing: Excipients are essential in the manufacturing of generic drugs, particularly when original drug patents expire. The introduction of new excipients can facilitate the reformulation of these drugs to comply with regulatory standards. This not only maintains the efficacy and stability of the drug but also enhances it. By integrating excipients, manufacturers can ensure that generic drugs remain a viable and effective option for treatment, keeping them accessible to patients.
- Drug Reformulation: In the pharmaceutical industry, the reformulation of drugs is a common strategy to enhance therapeutic effectiveness or extend market exclusivity. Excipients play a pivotal role in these processes. They enable modifications to drug delivery mechanisms and stability profiles, ensuring that reformulated drugs meet higher standards of efficacy and patient safety. This strategic use of excipients helps pharmaceutical companies maintain a competitive edge by improving existing drugs.
- Solubility Enhancement: Many modern drugs encounter solubility issues due to their intricate chemical structures, which can hinder their effectiveness. Excipients are crucial in addressing these challenges. They improve the solubility of active pharmaceutical ingredients, ensuring that drugs reach the necessary concentration in the body to provide effective treatment. This use of excipients is vital for maximizing the therapeutic impact of drugs, particularly those with complex formulations.
- Sustainability Practices: The development of excipients is increasingly aligned with sustainability goals in the pharmaceutical sector. This involves designing excipients that are produced with fewer resources and support environmentally friendly drug manufacturing practices. By focusing on sustainable excipients, the industry contributes to reducing the environmental impact of drug production. This shift not only helps companies meet regulatory requirements but also appeals to a growing segment of eco-conscious consumers.
Conclusion
The global pharmaceutical excipients market is poised for substantial growth, driven by advancements in drug formulation and manufacturing technologies. These excipients are crucial for enhancing the delivery, stability, and effectiveness of drugs, contributing significantly to the industry’s innovation. With stringent quality controls and evolving regulatory frameworks, the market is becoming more sophisticated, ensuring safety and efficiency in drug production. The adoption of new manufacturing processes and the integration of advanced technologies like nanotechnology and 3D printing are set to further revolutionize the sector. As the industry continues to adapt and embrace new challenges, the future of pharmaceutical excipients looks promising, marked by continuous improvement and regulatory advancements.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



