Table of Contents
Overview
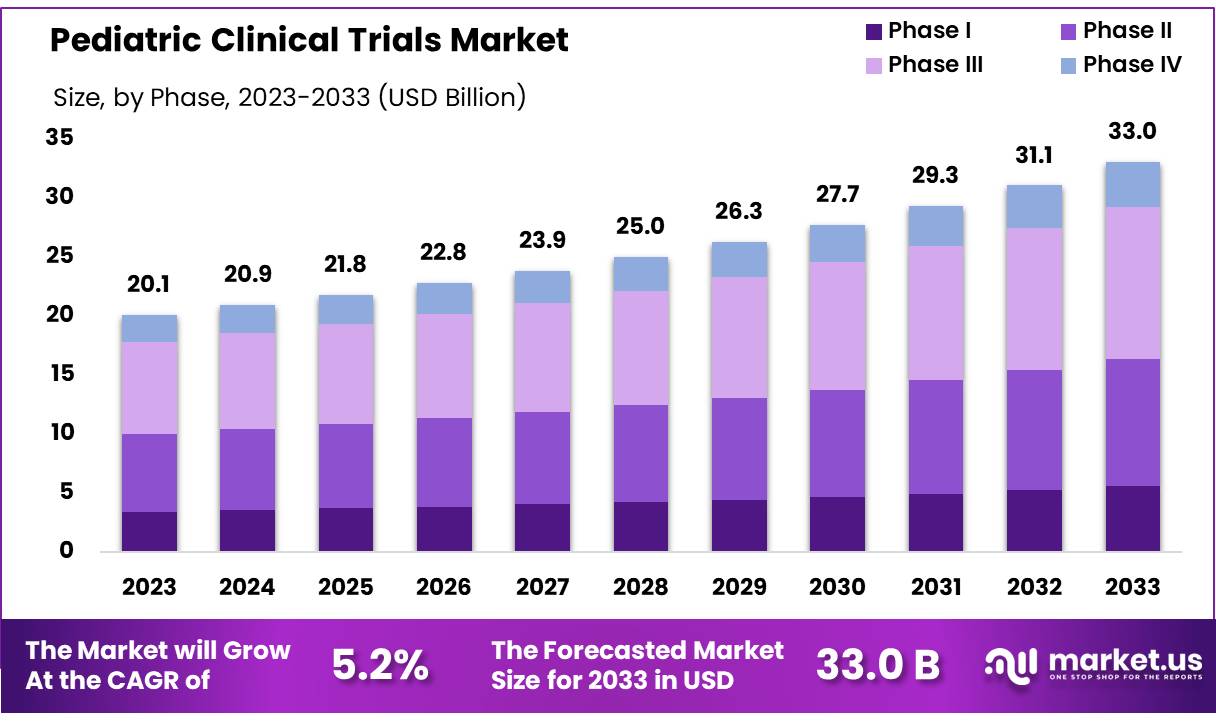
New York, NY – Sep 26, 2025 – Global Pediatric Clinical Trials Market size is expected to be worth around US$ 33.0 Billion by 2033 from US$ 20.9 Billion in 2024, growing at a CAGR of 5.0% during the forecast period from 2025 to 2033. In 2023, North America led the market, achieving over 39.10% share with a revenue of US$ 7.89 Billion.
The initiation of pediatric clinical trials marks a significant step in advancing safe and effective treatment options for children. These trials are designed to evaluate innovative therapies specifically tailored to meet the unique physiological needs of pediatric patients, ensuring that children benefit from the same scientific rigor applied in adult clinical research.
The pediatric clinical trial framework follows internationally accepted guidelines, with strict ethical oversight to safeguard young participants. Each study is carefully designed to minimize risks while maximizing the potential for medical breakthroughs. By involving leading pediatricians, researchers, and regulatory experts, the trials aim to generate high-quality evidence that supports safe dosage, efficacy, and long-term health outcomes.
Key therapeutic areas under study include rare genetic disorders, infectious diseases, oncology, and chronic conditions such as asthma and diabetes. The inclusion of diverse patient groups further strengthens the reliability and applicability of research findings.
Collaboration with healthcare providers, parents, and patient advocacy organizations plays an essential role in the success of these trials. Their engagement ensures transparency, trust, and adherence to the highest standards of patient care.
The launch of these pediatric trials represents a critical milestone toward building a healthier future for children worldwide. Results from the studies will provide actionable insights to advance regulatory approvals and expand access to innovative medicines designed for the youngest members of society.
Key Takeaways
- The Pediatric Clinical Trials market recorded revenues of US$ 20.10 billion and is projected to reach US$ 33.04 billion, expanding at a CAGR of 5.2% during the forecast period.
- By Phase, the Phase III segment dominated the market, accounting for a 38.9% share of total revenues.
- In terms of study design, the Treatment Studies segment held the largest contribution, representing 74.2% of the market share.
- Based on indication, the Infectious Diseases segment emerged as the leading category, securing a 25.2% share.
- By sponsor type, the industry segment led the market in 2023, capturing 52.7% of overall revenue.
- North America remained the top contributor, holding the largest share at 39.1% of the global market.
Regional Analysis
In 2023, North America dominated the global pediatric clinical trials market, accounting for 39.1% of total revenue. This leadership is largely driven by the strong presence of established industry players and the rising volume of clinical trials conducted across the region.
The region’s advanced healthcare infrastructure, including state-of-the-art research facilities and an extensive network of hospitals and clinics, ensures efficient trial execution and effective recruitment of pediatric participants.
Within North America, the United States is expected to remain a key growth hub, supported by substantial research and development investments from both government agencies and private organizations. These initiatives are fostering innovation and accelerating progress in pediatric therapies.
Moreover, increasing government support and favorable policies have encouraged pharmaceutical companies to expand their investments in pediatric trials within the region. Collectively, these factors reinforce North America’s dominant position and establish a strong platform for sustained market growth.
Frequently Asked Questions on Pediatric Clinical Trials
- What are pediatric clinical trials?
Pediatric clinical trials are research studies that evaluate the safety and effectiveness of medicines, vaccines, and treatments in children. These trials are essential to ensure therapies are safe, properly dosed, and effective for infants, children, and adolescents. - Why are pediatric clinical trials important?
Pediatric trials are vital because children often respond differently to medications compared to adults. Conducting these studies helps establish accurate dosage, minimizes adverse effects, and ensures children receive the best possible medical care tailored to their needs. - How are children protected in these trials?
Children’s safety in clinical trials is safeguarded through strict ethical guidelines, parental consent, and close medical monitoring. Institutional review boards and regulatory agencies oversee trial design to ensure risks are minimized and potential benefits are prioritized. - Who can participate in pediatric clinical trials?
Eligibility for pediatric clinical trials depends on age, medical condition, and study requirements. Children are usually recruited based on specific health criteria, and participation requires informed consent from parents or guardians, along with child assent where appropriate. - What is the pediatric clinical trials market?
The pediatric clinical trials market encompasses organizations, pharmaceutical companies, and research institutions conducting studies to develop child-focused therapies. It involves investments, regulatory frameworks, and partnerships aimed at advancing safe, effective treatments for pediatric populations worldwide. - What factors drive market growth?
Market growth is driven by rising prevalence of pediatric diseases, increasing demand for child-specific therapies, government incentives, and regulatory mandates for pediatric drug testing. Advancements in trial design and increased R&D funding further contribute to market expansion. - Which therapeutic areas dominate the market?
The pediatric clinical trials market is dominated by oncology, infectious diseases, vaccines, and rare genetic disorders. These areas receive significant research focus due to high disease burden among children and unmet medical needs requiring specialized therapeutic interventions. - What regions lead the market?
North America leads the pediatric clinical trials market due to strong regulatory support, advanced healthcare infrastructure, and high R&D investments. Europe follows closely, while Asia-Pacific is expected to witness rapid growth owing to expanding healthcare access and trial outsourcing.
Conclusion
The pediatric clinical trials market is witnessing steady growth, driven by rising demand for child-specific therapies, regulatory support, and increasing research investments. These trials ensure that children receive safe and effective treatments tailored to their unique physiological needs.
In 2023, the market generated US$ 20.10 billion, projected to reach US$ 33.04 billion by 2032 at a CAGR of 5.2%. North America leads due to robust healthcare infrastructure and R&D spending. Key therapeutic areas include infectious diseases, oncology, and genetic disorders. Strong collaborations among stakeholders will further advance pediatric care and accelerate access to innovative medicines worldwide.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



