Table of Contents
Overview
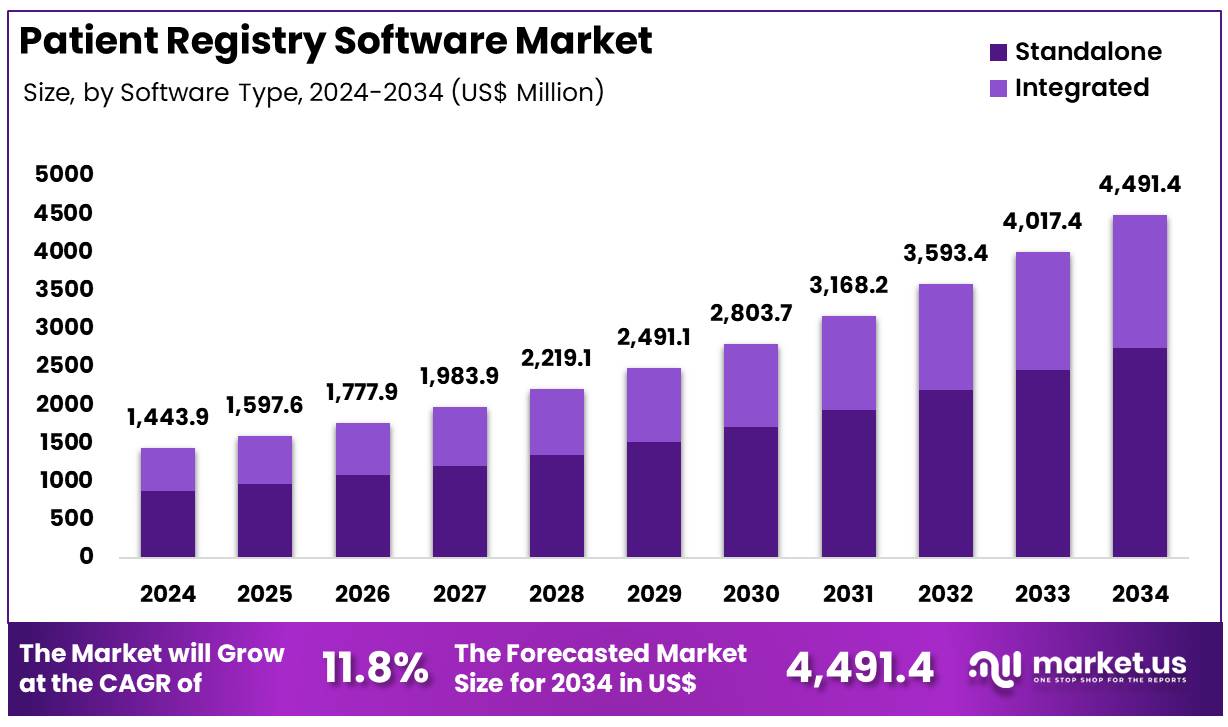
New York, NY – Nov 12, 2025 – Global Patient Registry Software Market was valued at US$ 1443.9 Million in 2024 and is expected to grow at a CAGR of 11.8% from 2024 to 2034. In 2024, North America led the market, achieving over 39.2% share with a revenue of US$ 566.0 Million.
A new Patient Registry Software has been introduced to enhance the collection, management, and analysis of patient data across healthcare systems. The software is designed to streamline clinical data aggregation, improve patient outcomes, and support evidence-based decision-making.
This advanced digital platform enables healthcare providers, research institutions, and pharmaceutical companies to efficiently manage large volumes of patient information in compliance with regulatory standards such as HIPAA and GDPR. The software facilitates real-time data entry, customizable registry design, and seamless integration with electronic health records (EHRs).
By offering powerful analytics tools, the system supports clinical trials, post-market surveillance, and long-term patient monitoring. Its intuitive interface allows stakeholders to track disease trends, treatment effectiveness, and patient safety outcomes with greater accuracy.
The Patient Registry Software aims to bridge data gaps within healthcare ecosystems, fostering collaboration among hospitals, research centers, and government health agencies. It also promotes interoperability and transparency in clinical research and population health management.
According to the company spokesperson, the launch marks a significant step toward digital transformation in healthcare, ensuring that patient-centric data is securely captured and utilized for better medical insights. The solution is now available globally for healthcare institutions seeking to modernize their data infrastructure.
Key Takeaways
- The global patient registry software market was valued at USD 1,443.9 million in 2024 and is projected to reach USD 4,491.4 million by 2034, expanding at a compound annual growth rate (CAGR) of 11.8% during the forecast period.
- In 2024, the disease registry segment dominated the market, accounting for 62.9% of the total revenue share.
- The standalone software segment emerged as the leading deployment type, capturing 61.3% of the overall market share in 2024.
- The on-premise deployment model held the largest share of 69.5%, reflecting continued preference for in-house data management solutions.
- Based on end user, the commercial segment led the market, representing 63.5% of the total revenue share in 2024.
- North America retained its dominance in the global patient registry software market, contributing to over 39.2% of the total revenue in 2024.
Patient Registry Software Statistics
- Scope and Reach: As of January 2024, there are approximately 70 active registry sites across North America, reflecting the growing network and wide adoption of patient registry systems in the healthcare ecosystem.
- Enrollment and Data Collection: More than 14,000 participants have been enrolled in various registries, with over 90,000 clinical visits recorded. This highlights the capacity of these platforms to support large-scale and longitudinal data collection efforts.
- Biosample Integration: Over 1,700 biosamples have been successfully integrated with registry data, significantly enhancing data depth and supporting more comprehensive clinical and translational research outcomes.
- Clinical Trials and Studies: Patient registries play a vital role in research activities, supporting 3 Phase 4 studies and 15 active or completed cohort studies utilizing CARRA Registry data. Additionally, a Phase 3 layered clinical trial is currently underway, demonstrating ongoing research applications.
- Specific Initiatives:
- HERO Registry: Established in April 2020, the registry enrolled over 50,000 members, demonstrating exceptional capacity for rapid data acquisition.
- HERO-Together: Supported by Pfizer, this initiative was launched alongside the first COVID-19 vaccine EUA and enrolled over 20,000 members to evaluate vaccine impacts.
- HERO-BOOST: Focuses on studying newer, bivalent COVID-19 vaccines, with over 10,000 consented participants, emphasizing the registry’s continued relevance in post-pandemic research.
- Financial Support: Funding opportunities provide up to USD 150,000 per year for two years, with an additional 12% for indirect costs, reflecting the strong financial backing for registry-based research initiatives.
- Demonstration of Clinical Efficacy: For rare conditions such as Duchenne Muscular Dystrophy, where trials may involve fewer than 20 patients, registries deliver essential clinical efficacy data. These insights support regulatory evaluations and influence payer reimbursement strategies.
Regional Analysis
The patient registry software market in North America is experiencing robust growth, driven by the increasing focus on healthcare data management. The regional healthcare sector is prioritizing efficient data collection, integration, and analysis to enhance patient outcomes, optimize treatment pathways, and reduce operational costs. Patient registry software serves as a critical component in achieving these goals by centralizing and organizing patient information, thereby enabling healthcare providers to make data-driven clinical decisions.
Furthermore, the development of Health Information Exchanges (HIEs) across several U.S. states and Canadian provinces has accelerated the adoption of patient registry solutions. These exchanges promote seamless data sharing among healthcare systems and authorized professionals, ensuring comprehensive and accessible patient records. The integration of patient registry software within HIE frameworks significantly contributes to the expansion of the regional market.
A 2022 study titled “How A Clinical Data Registry Can Work With Your EHR” highlighted that data captured within clinical registries supports healthcare professionals in identifying the most effective treatment strategies for patients with similar medical conditions. This underscores the growing importance of patient registry systems as foundational tools in advancing evidence-based care across North America.
Emerging Trends in Patient Registry Software
- AI and Machine Learning Integration: The incorporation of artificial intelligence (AI) and machine learning (ML) is enhancing data analytics within registries, enabling predictive modeling and personalized treatment strategies for improved patient outcomes.
- Emphasis on Patient-Reported Outcomes: There is an increasing focus on collecting patient-reported outcomes, allowing registries to capture real-world feedback on health status and treatment satisfaction, making systems more patient-centric.
- Expansion of Global Registries: The growth of multinational registries is broadening the understanding of diseases across diverse populations, resulting in globally relevant and generalizable research findings.
- Data Security and Privacy: With rising digitalization, data protection has become a core priority. Technologies such as encryption and multi-factor authentication are being widely implemented to safeguard sensitive patient information.
- Adoption of Cloud-Based Solutions: Cloud platforms are increasingly preferred for their scalability, flexibility, and cost-efficiency, enabling remote access, simplified maintenance, and improved data interoperability across systems.
- Telehealth Integration: Electronic medical record (EMR) systems are now incorporating telehealth functionalities, expanding healthcare accessibility and supporting remote patient monitoring and consultations.
- Big Data Analytics: The integration of big data analytics within registries allows deeper insights into disease trends, treatment responses, and public health outcomes, enhancing evidence-based policymaking.
- Enhanced Usability for Providers: Developments are focused on improving user experience through simplified interfaces, customizable workflows, and intuitive navigation, optimizing clinical efficiency.
- Longitudinal Studies and Tracking: Registries facilitate long-term studies, enabling continuous monitoring of disease progression and treatment safety over extended periods.
- Innovative Data Collection Methods: The use of mobile health apps and wearable devices is expanding, adding real-time, patient-generated health data that enrich registry datasets and improve research precision.
Frequently Asked Questions on Patient Registry Software
- How does patient registry software benefit healthcare providers?
The software helps healthcare providers centralize patient information, identify care gaps, and track treatment effectiveness. It supports better disease management, facilitates clinical research, and enhances overall healthcare efficiency through structured data insights. - What are the key features of patient registry software?
Key features include customizable registry templates, real-time data entry, interoperability with electronic health records (EHRs), secure data storage, analytical dashboards, and automated reporting for research and clinical management. - Who uses patient registry software?
Hospitals, research institutions, government agencies, and pharmaceutical companies use patient registry software to collect and analyze patient outcomes, monitor treatment patterns, and support clinical studies. - How does patient registry software ensure data security?
The software employs encryption, access controls, and compliance with standards like HIPAA and GDPR to safeguard sensitive health information against unauthorized access and data breaches. - Which regions dominate the patient registry software market?
North America dominates the global market due to advanced healthcare infrastructure, government initiatives supporting data interoperability, and widespread implementation of electronic health records. - What are the major segments of the patient registry software market?
The market is segmented by type, deployment, and end user. Key segments include disease registries, standalone systems, and on-premise deployment models serving hospitals and commercial entities. - What is the future outlook for the patient registry software market?
The market is expected to witness significant growth, driven by digital transformation in healthcare, increasing demand for real-world evidence, and investments in advanced data analytics technologies.
Conclusion
In conclusion, the global patient registry software market is witnessing rapid expansion, driven by the growing need for efficient healthcare data management, clinical research, and evidence-based decision-making. The increasing adoption of digital technologies, integration of AI and cloud solutions, and focus on patient-centered outcomes are transforming registry systems into essential tools for modern healthcare.
With strong regional growth in North America, robust funding support, and advancements in data security and interoperability, patient registry software is poised to play a pivotal role in enhancing healthcare quality, supporting research initiatives, and enabling long-term, data-driven improvements in patient care.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



