Table of Contents
Overview
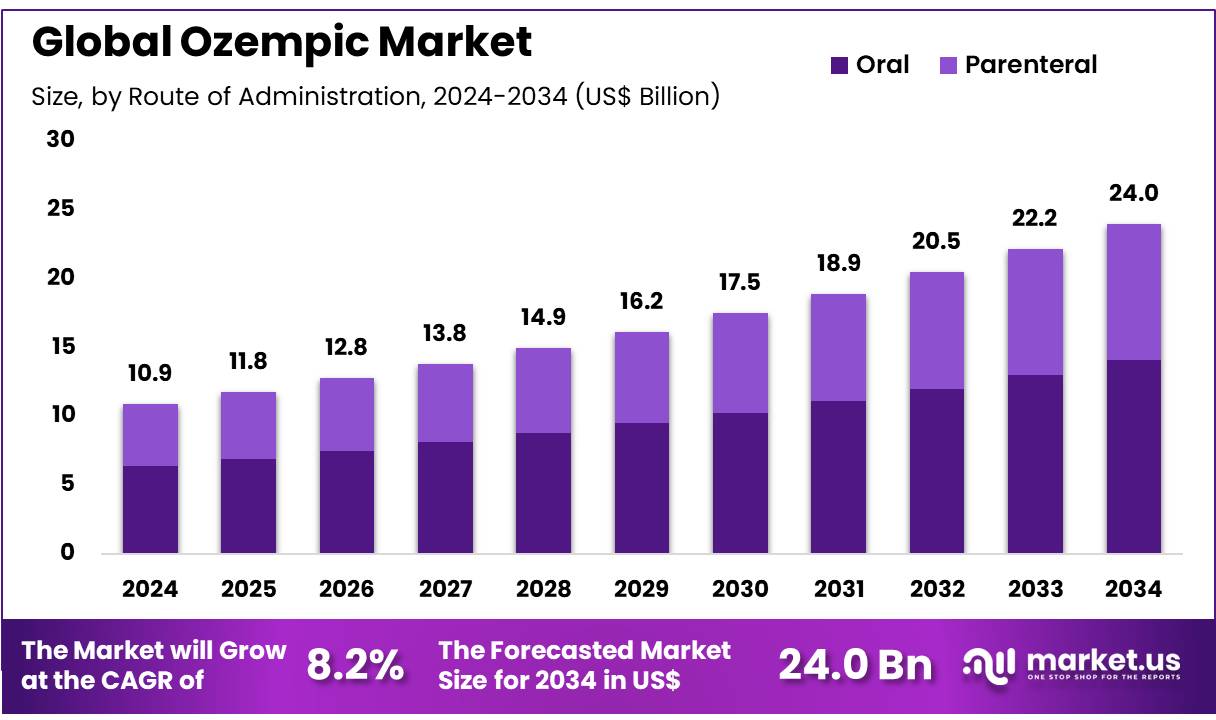
New York, NY – Nov 07, 2025 – Global Ozempic Market size is expected to be worth around US$ 24.0 Billion by 2034 from US$ 8.2% Billion in 2024, growing at a CAGR of 8.2% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 42.3% share with a revenue of US$ 4.6 Billion.
A clear increase in global interest has been observed in glucagon-like peptide-1 (GLP-1) receptor agonists, and Ozempic has been recognized as one of the leading products within this category. Ozempic is a once-weekly injectable therapy developed to support improved glycemic control in adults with type 2 diabetes.
The product operates through the modulation of insulin secretion and the reduction of glucagon release, leading to more stable blood glucose levels. Its mechanism of action is aligned with physiological pathways that regulate appetite and metabolic function, which has resulted in additional attention from healthcare stakeholders.
The adoption of Ozempic has been supported by clinical data indicating reductions in glycated hemoglobin levels, with improvements often ranging between 1.0 percent and 1.5 percent from baseline in key studies. The product has also been associated with moderate weight reduction in many individuals, although outcomes vary. The growth of its global market presence can be attributed to rising diabetes prevalence, heightened awareness of GLP-1 therapies, and increasing emphasis on long-term disease management.
Ozempic is currently approved in multiple regions and is positioned as part of a comprehensive treatment plan that includes diet and physical activity. The product’s safety profile is generally consistent with the GLP-1 class, with gastrointestinal events being the most commonly reported. Continued demand is expected as healthcare systems prioritize innovative metabolic therapies and as ongoing research expands the understanding of GLP-1-based treatment pathways.
Key Takeaways
- The market for Ozempic generated revenue of US$ 8.2 billion in 2024, and growth has been projected at a CAGR of 8.2%, with the market expected to attain US$ 24.0 billion by 2033.
- The route of administration has been segmented into oral and parenteral, with the oral segment accounting for 58.7% of the market share in 2023.
- The distribution channel includes hospital pharmacies, retail pharmacies, and online pharmacies, with hospital pharmacies holding 46.8% of the overall share.
- North America led the global market in 2023 by capturing a 42.3% share.
Regional Analysis
North America is Leading the Ozempic Market
North America accounted for the largest share of 42.3% due to the rising prevalence of type 2 diabetes and increasing awareness of GLP-1 receptor agonists for effective glycemic management. As reported in the CDC’s National Diabetes Statistics Report 2022, more than 130 million adults in the United States are affected by diabetes or prediabetes, with underserved populations experiencing a higher disease burden because of healthcare inequities.
The adoption of Ozempic continued to expand as its use grew in both diabetes treatment and weight management. Prescriptions increased as healthcare professionals recognized its combined benefits of blood sugar control and weight reduction, which strengthened its market presence. Direct-to-consumer promotional efforts and physician endorsements contributed to higher patient awareness, while broader insurance coverage and reimbursement support enhanced accessibility.
Growing clinical evidence on the cardiovascular benefits associated with GLP-1 receptor agonists further supported acceptance among physicians and patients, reinforcing the region’s demand for advanced therapies for diabetes management.
The Asia Pacific Region Is Expected to Record the Highest CAGR
Asia Pacific is projected to register the fastest growth rate due to the increasing number of type 2 diabetes cases and improving healthcare access. Rapid urbanization and lifestyle changes in major countries such as China, India, and Japan are contributing to rising obesity and diabetes levels, which are expected to increase demand for modern treatment options. Government-led initiatives aimed at improving diabetes awareness and encouraging early diagnosis are likely to support higher prescription volumes.
Partnerships between global pharmaceutical companies and regional healthcare stakeholders are expected to enhance product availability and affordability. Growth in the middle-class population and rising healthcare expenditure will further support adoption. Expanding reimbursement coverage for diabetes management across several countries is anticipated to improve patient access.
In addition, increasing use of digital health platforms and telemedicine for chronic disease monitoring is expected to facilitate wider treatment uptake, thereby supporting overall market expansion in the Asia Pacific region.
Emerging Trend
- Expansion of Clinical Research Beyond Diabetes: Semaglutide is under investigation for multiple new indications, including alcohol use disorder, early Alzheimer’s disease, and cardiovascular disease. SELECT trial findings demonstrated reduced cardiovascular events among 17,604 obese participants, while additional studies in kidney disease, NASH, idiopathic intracranial hypertension, and sleep apnea indicate broadening therapeutic relevance.
- Advancements in Combination Therapies: Combination regimens such as cagrilintide plus semaglutide have shown greater weight-loss efficacy than semaglutide alone. REDEFINE trials reported reductions up to 20.4 percent over 68 weeks, compared with 14.9 percent for semaglutide. These results suggest a future shift in clinical practice toward dual GLP-1–based therapies.
- Impact of Patent Expiry and Pricing Pressures: Market access is being reshaped by approaching patent expirations and Medicare pricing reforms. U.S. patents extend to 2031, while China and Brazil face earlier expirations. Medicare spending increases triggered Inflation Reduction Act negotiations, and lower reimbursement is expected by 2027. FDA warnings highlight concerns regarding counterfeit pens.
Use Cases
- Type 2 Diabetes Management: Ozempic’s 2017 FDA approval was based on seven global trials involving 4,087 patients, demonstrating strong glycemic and cardiovascular outcomes. Weekly semaglutide reduced HbA1c by up to 1.5 percentage points, with 20–40 percent achieving fasting glucose below 130 mg/dL, supporting its role in long-term diabetes control.
- Obesity and Weight Management: Semaglutide has gained widespread off-label use for weight loss, supported by clinical evidence showing 14 percent reductions in phase II trials. SELECT data demonstrated 15–20 percent weight loss in obese adults without diabetes. By mid-2025, millions of Medicare and Medicaid beneficiaries may qualify for GLP-1 obesity-drug coverage.
- Cardiovascular Risk Reduction: Semaglutide has demonstrated meaningful cardiovascular protection in high-risk obese adults. In the SELECT trial, 17,604 participants followed for nearly 40 months showed event reductions from 8.0 percent with placebo to 6.5 percent with treatment. These outcomes guide considerations for semaglutide use beyond diabetes management.
- Emerging and Off-Label Indications: Ongoing research is assessing semaglutide for alcohol use disorder, Alzheimer’s disease, chronic kidney disease, and NASH. Early findings indicate reduced alcohol intake, potential cognitive benefits, slower renal decline, and significant NAFLD score improvements. These investigations highlight semaglutide’s expanding therapeutic potential across metabolic and organ-specific conditions.
Frequently Asked Questions on Ozempic
- How does Ozempic work?
The mechanism of action involves stimulation of GLP-1 receptors, resulting in increased insulin secretion, reduced glucagon release, and delayed gastric emptying. These combined effects support improved glycemic outcomes and gradual weight loss among individuals diagnosed with type 2 diabetes. - What are the primary benefits of Ozempic?
The benefits include stable glucose control, reduced HbA1c levels, and moderate weight loss, which collectively contribute to improved metabolic health. Cardiovascular risk reduction has also been observed, strengthening the product’s acceptance among clinicians managing long-term diabetes complications. - Who is prescribed Ozempic?
Ozempic is prescribed for adults with type 2 diabetes when lifestyle changes or oral therapies provide insufficient control. Its use is often considered for patients requiring improved glycemic stability and incremental cardiovascular protection under physician-supervised treatment plans. - Are there any common side effects of Ozempic?
The product’s safety profile includes gastrointestinal effects such as nausea, vomiting, and diarrhea, which are typically mild and temporary. These reactions tend to decline as the patient’s system adjusts, supporting consistent adherence to ongoing therapeutic regimens. - Is Ozempic used for weight loss?
Although not primarily approved as a weight-loss drug, Ozempic has been associated with notable weight reduction in diabetic patients. This effect is attributed to appetite regulation, slower gastric emptying, and improved metabolic efficiency observed during clinical use. - Which regions dominate the Ozempic market?
North America maintains the largest share due to its high diabetes burden, strong healthcare infrastructure, and reimbursement availability. Europe follows closely, while rapid adoption across Asia Pacific is expected due to expanding patient populations and improved diagnostic efforts. - Who are the major players in the Ozempic market?
Novo Nordisk is the leading manufacturer, supported by strong research investments and global distribution networks. Competitive pressure is expected to increase as other companies expand GLP-1 pipelines and explore novel formulations to capture emerging therapeutic opportunities.
Conclusion
The global Ozempic market is expected to continue expanding as demand for advanced metabolic therapies increases and evidence supporting GLP-1 receptor agonists strengthens. Growth is being driven by rising diabetes prevalence, broader use in weight management, and expanding clinical research across multiple therapeutic areas.
North America retains market leadership, while Asia Pacific is projected to record the fastest growth due to improving healthcare access and rising disease burdens. Advancements in combination therapies and ongoing innovation are expected to further enhance adoption. Despite pricing pressures and upcoming patent expirations, long-term demand remains strong as healthcare systems prioritize effective chronic disease management.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



