Table of Contents
Introduction
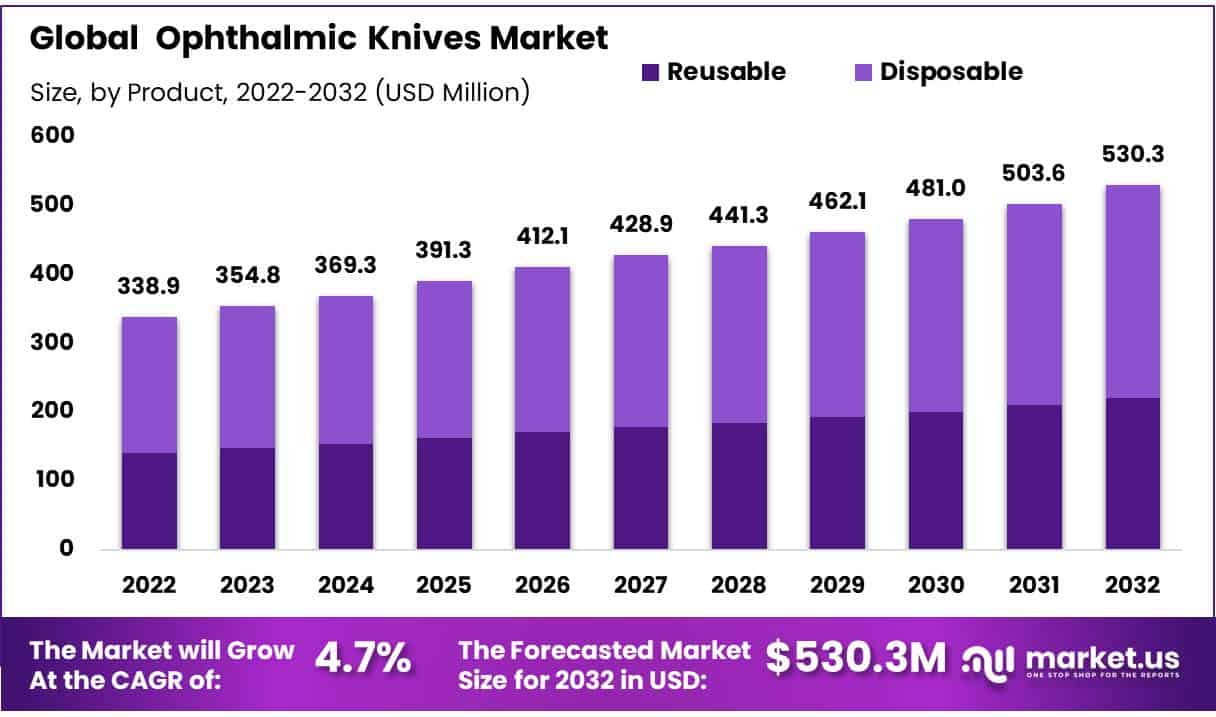
The Global Ophthalmic Knives Market is expected to reach USD 530.3 million by 2032, up from USD 354.9 million in 2023, growing at a CAGR of 4.7% during the forecast period from 2023 to 2032. This growth is driven by several key factors, including the increasing prevalence of eye diseases requiring surgical intervention, such as cataracts and glaucoma. The CDC reports a rising incidence of these conditions, particularly among the aging population, which increases the demand for ophthalmic surgical tools.
Technological advancements in ophthalmic devices also contribute significantly to market growth. Innovations in the precision and safety of surgical knives enhance surgical outcomes and reduce complications, making these tools more desirable in ophthalmic surgeries. Additionally, regulatory support and quality control standards established by the FDA ensure the safety and effectiveness of ophthalmic knives, boosting market confidence and adoption.
The ophthalmic knives market has seen significant developments recently, driven by strategic acquisitions, product launches, and mergers by leading companies. For instance, in May 2024, Alcon Vision LLC acquired a specialized ophthalmic knife manufacturer in Germany. This acquisition aims to enhance Alcon’s surgical instruments portfolio and improve precision in ophthalmic surgeries, thereby solidifying their market position.
Key Takeaways
- Market Size: The Global Ophthalmic Knives Market is expected to reach USD 530.3 million by 2032, up from USD 354.9 million in 2023.
- Market Growth: The Global Ophthalmic Knives Market is growing at a CAGR of 4.7% during the forecast period from 2023 to 2032.
- Product Analysis: In 2023, disposable ophthalmic knives held a dominant market share, accounting for 58.3% of the total market.
- Design Analysis: The straight knives segment is projected to maintain a significant share of 38% from 2023 to 2032.
- Application Analysis: The category of cataract surgery led the market in terms of application, highlighting the importance of these tools in such procedures.
- End-User Analysis: Hospitals generated the highest revenue in the Ophthalmic Knives Market in 2022, underscoring their critical role as primary end-users.
- Regional Analysis: North America held the largest share of the ophthalmic knife market, accounting for 35% in 2022.
- Clinical Relevance: Ophthalmic knives are essential in eye surgeries, reducing patient trauma, accelerating recovery times, and improving post-operative vision outcomes.
- Quality Assurance: Adherence to strict quality control measures and regulatory standards ensures the safety and efficacy of ophthalmic knives.
- Market Drivers: The market is expanding due to several factors, including the increasing prevalence of eye disorders, advancements in surgical techniques, and a growing elderly population.
Ophthalmic Knives Statistics
- Procedure Volume: Annually, approximately 3 million cataract surgeries are performed in the U.S., with ophthalmic knives playing a crucial role in these procedures.
- Cost: The average cost of a high-quality disposable ophthalmic knife ranges from $15 to $50 per unit, depending on the specifications.
- Global Sales: The global sales of ophthalmic knives account for about 20% of the total ophthalmic surgical instruments market.
- Usage: Over 90% of ophthalmologists in developed countries use disposable knives to minimize infection risk.
- Surgical Precision: Ophthalmic knives enable precision cuts as small as 1.2 mm, critical for minimally invasive eye surgeries.
- Advancements: 50% of new ophthalmic knife designs focus on ergonomic improvements to reduce surgeon fatigue.
- Material: Approximately 60% of ophthalmic knives are made from stainless steel due to its durability and sharpness.
- Cataract Surgery: Ophthalmic knives are used in 100% of cataract surgeries, which are the most common type of eye surgery.
- Sterilization: Disposable knives are preferred in 85% of surgeries to avoid sterilization challenges.
- Innovation: 30% of research and development in ophthalmic instruments focuses on improving blade sharpness and durability.
- Market Share: The North American region holds about 40% of the global market share for ophthalmic knives.
- Environmental Impact: The industry is exploring biodegradable materials for knives, with a goal to reduce plastic waste by 20% by 2030.
Companies Recent Developments
- In April 2024, Bausch & Lomb Incorporated introduced the PrecisionEdge Plus, an advanced ophthalmic knife designed to provide superior sharpness and control. This product aims to improve surgical outcomes in delicate eye procedures, highlighting the company’s commitment to innovation in ophthalmic surgery. Similarly, BVI completed a merger with a leading microsurgical tool company in March 2024, intending to expand their product range and enhance their technological capabilities in the ophthalmic surgery market.
- In February 2024, Diamatrix Ltd. launched the MicroBlade Ultra, a new line of ophthalmic knives offering enhanced precision and durability for ophthalmic surgeons. This product addresses the increasing demand for high-quality surgical tools. Additionally, in January 2024, HAI Laboratories, Inc. acquired a pioneering ophthalmic instrument company. This acquisition is part of their strategy to broaden their product offerings and strengthen their market position in ophthalmic surgical instruments.
- In April 2024, Surgistar, Inc. merged with an innovative surgical technology firm. This merger focuses on integrating cutting-edge technologies to develop next-generation ophthalmic knives, enhancing precision and efficiency in eye surgeries. These developments reflect the dynamic nature of the ophthalmic knives market, with leading companies continuously investing in advancements to improve surgical outcomes and meet the growing demand for precise and efficient ophthalmic tools.
Emerging Trends
- Regulatory Classification: Ophthalmic knives are classified under manual surgical instruments within regulatory frameworks like the FDA’s. As Class I devices, they must meet basic safety and effectiveness standards, ensuring they are suitable for their intended use in ophthalmic procedures. This classification requires adherence to fundamental regulations but often exempts devices from more extensive premarket notification processes, focusing instead on maintaining essential performance and safety standards.
- Recall and Quality Control: The importance of quality control in ophthalmic knives is underscored by occasional recalls, such as those initiated by companies like Alcon due to issues with blade sharpness. These recalls highlight the necessity of rigorous quality assurance processes. Ensuring that each knife meets high standards of precision is critical, as even minor defects can significantly impact surgical outcomes. Manufacturers are therefore investing in enhanced quality control measures to prevent such issues and maintain high standards.
- Product Classification and Standards: The FDA categorizes ophthalmic knives under product code HNN, which includes specific guidelines for their premarket review and compliance. Although some knives are exempt from certain premarket notification requirements, they must still adhere to stringent standards to ensure their safety and effectiveness. These guidelines help standardize the performance and reliability of ophthalmic knives, contributing to their overall efficacy in surgical procedures.
- Ophthalmic Medical Technicians: Ophthalmic medical technicians play a vital role in the successful use of ophthalmic knives during surgeries. They assist in various tasks, from preparing the instruments to ensuring their proper handling and application. Their expertise is essential in maintaining the precision and safety of the surgical process, directly impacting patient outcomes. The demand for skilled ophthalmic technicians is growing as the complexity of ophthalmic procedures increases.
- Geographic Distribution: The demand for ophthalmic medical technicians, who work closely with ophthalmic knives, is notably high in states like Florida, Texas, and California. This regional concentration reflects the high volume of ophthalmic surgeries and the need for specialized instruments and expertise in these areas. These states are hubs for ophthalmic practices and surgical centers, driving the demand for both advanced surgical tools and skilled professionals.
- Training and Expertise: Continuous training for healthcare professionals using ophthalmic knives is crucial. Ongoing education ensures that surgeons and technicians stay updated with the latest techniques and safety protocols. Proper training minimizes the risks associated with the use of these instruments, enhancing surgical precision and patient safety. Regulatory bodies emphasize this training as a key component in maintaining high standards of care.
- Focus on Safety: Safety and sterility are paramount in the use of ophthalmic knives. The trend towards disposable knives reflects a growing focus on reducing the risk of infections and ensuring patient safety. Disposable knives help mitigate the risks associated with reuse and are increasingly preferred in clinical settings to maintain high standards of hygiene and patient care.
- Technological Innovation: The ophthalmic knives market is witnessing advancements in blade design, such as the incorporation of diamond-coated blades that offer enhanced precision. These innovations contribute to improved surgical outcomes and are subject to regulatory oversight to ensure they meet safety standards. Ongoing technological improvements aim to refine the performance of ophthalmic knives, supporting the advancement of surgical techniques and enhancing overall efficacy.
Use Cases
- Regulatory Standards and Safety: Ophthalmic knives are categorized as Class 2 medical devices, subject to stringent regulatory standards to ensure their safety and effectiveness. Guidelines for labeling and recalls are enforced to address any issues related to the sharpness or quality of these knives promptly. This regulatory framework helps maintain high standards in device performance and patient safety.
- Surgical Precision: In eye surgeries such as cataract and glaucoma procedures, ophthalmic knives are indispensable due to their precision. These knives are designed to make extremely accurate incisions, minimizing tissue trauma and enhancing surgical outcomes. The precision offered by these tools is crucial for successful eye surgeries.
- Device Classification and Design: Ophthalmic knives come in various designs, including straight, crescent, and micro-phaco, each tailored for specific surgical needs. Typically crafted from stainless steel or diamond, these knives are chosen for their durability and precision. The variety in design allows surgeons to select the optimal tool for different types of procedures.
- Single-Use vs. Reusable Options: The market provides both single-use and reusable ophthalmic knives. Single-use knives are preferred in many settings to reduce the risk of infections, while reusable knives are valued for their cost-effectiveness and durability. The choice between single-use and reusable options often depends on the specific surgical environment and budget considerations.
- Risk Mitigation and Recalls: To ensure patient safety, regulatory bodies monitor ophthalmic knives closely and issue recalls if necessary. For example, voluntary recalls may occur if there are issues with sharpness or other quality concerns. Such measures are implemented to mitigate risks and uphold safety standards in surgical practices.
- Regulatory Compliance for Safety: Compliance with regulatory guidelines is essential for manufacturers of ophthalmic knives. This includes adhering to labeling requirements and undergoing regular inspections to prevent contamination and ensure the sterility of the instruments. Maintaining these standards is critical for the overall safety and quality of the knives.
- Technological Advancements: Technological innovations, such as the development of diamond-coated blades, have significantly enhanced the precision and effectiveness of ophthalmic knives. These advancements contribute to better surgical outcomes and are supported by regulatory bodies to ensure they meet high safety standards.
- Patient Safety and Infection Control: Emphasizing the importance of sterile instruments, regulatory guidelines stress the use of single-use ophthalmic knives to prevent infections during eye surgeries. This focus on infection control has led to a greater preference for disposable knives in various surgical settings, ensuring better patient safety and reducing the risk of complications.
Conclusion
The Global Ophthalmic Knives Market is projected to grow significantly, reaching USD 530.3 million by 2032, driven by the increasing prevalence of eye diseases such as cataracts and glaucoma. The aging population and technological advancements in ophthalmic devices further fuel this growth. Innovations in precision and safety of surgical knives enhance surgical outcomes, making these tools more desirable. Recent developments include strategic acquisitions and product launches by companies like Alcon Vision LLC and Bausch & Lomb, emphasizing their commitment to innovation. Regulatory standards and quality control measures ensure the safety and effectiveness of ophthalmic knives, boosting market confidence and adoption.


