Overview
New York, NY – August 19, 2025: The Global Onychomycosis Treatment Market is projected to grow from US$ 3.88 billion in 2024 to US$ 5.97 billion by 2034, advancing at a CAGR of 4.4%. North America remains the leading region, holding a 40.4% share, equivalent to US$ 1.57 billion in 2024. The market is driven by high prevalence of fungal nail infections, particularly among older adults, and rising healthcare awareness. The World Health Organization (WHO) and the CDC confirm that onychomycosis is common in ageing populations, often causing nail thickening, discoloration, and mobility limitations. This steady clinical need ensures continued demand for antifungal therapies.
Demographic ageing is a primary market driver. By 2030, one in six people globally will be aged 60 years or above. As infection risk increases with age, this demographic shift expands the patient pool for both oral and topical antifungals. Rising diabetes prevalence is another major factor. WHO reports that 830 million people were living with diabetes in 2022, while CDC guidance highlights their higher risk of fungal nail infections. These overlapping risk groups—older adults and diabetic patients—create sustained demand for effective treatment options.
Expanding High-Risk Populations
An expanding immunosuppressed population is strengthening market prospects. The WHO–ONT Global Observatory reported 172,409 organ transplants in 2023, an increase of 9.5% from 2022. Transplant recipients, along with other immunocompromised patients, face a higher risk of fungal infections. In addition, CDC guidance highlights compromised immunity as a critical risk factor. This enlarges the addressable patient base for antifungal therapies and raises the long-term importance of effective treatment interventions.
Regulatory and Competitive Landscape
Recent regulatory activity has broadened treatment options. The U.S. FDA approved topical agents efinaconazole and tavaborole in 2014, and generic versions of tavaborole topical solution were authorized in 2024, improving affordability. Greater price competition enhances access and volume uptake in this chronic and cosmetically significant condition. Meanwhile, antifungal resistance is emerging as a challenge. The CDC has reported rising terbinafine-resistant strains, including T. rubrum and T. indotineae, prompting research into new drug candidates, optimized dosing, and combination therapies.
Diagnostic and Healthcare Trends
Improved diagnostic guidance is shaping market expansion. CDC and Public Health England recommend laboratory confirmation using microscopy, culture, or molecular methods before systemic therapy. Better diagnostics reduce misdiagnosis and align treatment choices. In the United States, the CDC estimates millions of outpatient visits annually for fungal diseases, reflecting significant healthcare utilization. Growing attention to fungal disease management, along with a large persistent patient base, supports long-term growth for the onychomycosis treatment market.
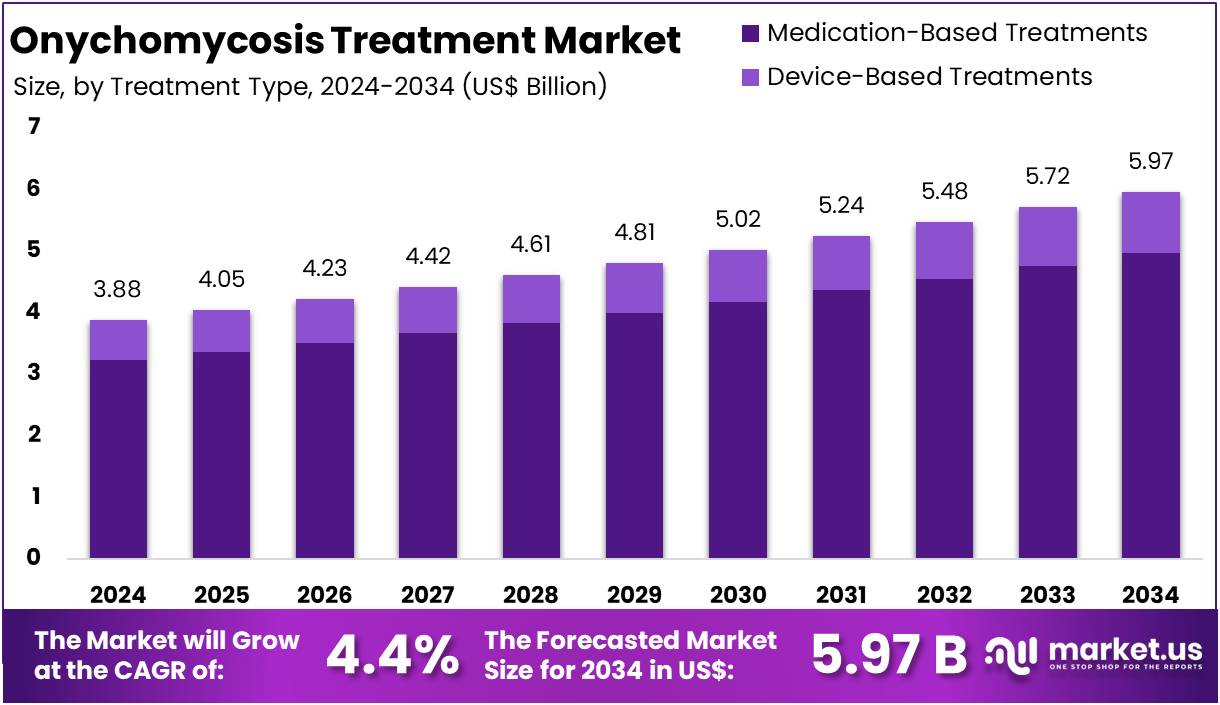
Key Takeaways
- In 2024, the Onychomycosis Treatment market earned US$ 3.88 billion revenue and is projected to reach US$ 5.97 billion by 2034.
- The market is growing at a CAGR of 4.4% between 2025 and 2034, reflecting consistent demand for effective fungal nail infection solutions.
- Medication-based treatments dominated with an 83.2% market share in 2024, while device-based treatments contributed the remaining portion of the treatment segment.
- Dermatophytes led the pathogen type category, accounting for 72.7% share in 2024, while yeasts and non-dermatophyte molds formed smaller market segments.
- Distal Subungual Onychomycosis (DSO) was the most common disease type, representing 44.8% of the market share in 2024 alone.
- Retail pharmacies were the leading distribution channel in 2024, holding 48.7% market share, surpassing online pharmacies and hospital or clinic-based sales.
- North America emerged as the dominant regional market, securing a 40.4% market share in 2024 due to high treatment adoption and awareness.
Regional Analysis
North America dominates the Onychomycosis Treatment Market with a strong 40.4% share. This leadership is supported by advanced healthcare systems, high patient awareness, and a sizable population affected by the condition. The United States plays the biggest role, driven by a higher prevalence of onychomycosis among older adults and diabetic patients. These groups are more vulnerable, which increases the demand for effective treatments. Access to quality care and early diagnosis also contributes to the region’s strong performance in the global market.
The presence of leading pharmaceutical companies further strengthens North America’s market position. Key players such as Bausch Health and Novartis drive growth by offering widely used oral antifungals and topical solutions. These medications are considered the first line of treatment, ensuring steady adoption. The focus on research and development, along with strong distribution networks, enhances availability. As a result, treatment rates remain high compared to other regions, supporting consistent revenue growth and expanding market penetration across the United States and Canada.
North America also benefits from easy access to dermatologists and podiatrists, which helps in timely treatment. The rising preference for advanced treatment options, including laser-based therapies, supports innovation in this market. Patients in the region are willing to try new technologies due to higher disposable incomes and better insurance coverage. Such factors ensure strong adoption of both conventional and modern solutions. This trend further cements North America’s role as a global leader in onychomycosis treatment, setting a benchmark for other regions to follow.
In December 2021, Bausch Health and its dermatology division, Ortho Dermatologics, achieved a key milestone. Their product JUBLIA (efinaconazole) Topical Solution, 10%, received the APMA Seal of Approval. This recognition highlighted its proven clinical effectiveness and safety in treating fungal nail infections. As JUBLIA is a leading prescription therapy in the U.S., the endorsement is expected to boost physician confidence. It may also drive greater adoption among patients and strengthen Bausch Health’s competitive position in the growing North American market.
Emerging Trends
- Oral terbinafine stays first choice: Oral terbinafine remains the leading treatment for nail fungus. A daily dose of 250 mg for 12 weeks delivers high success. Trials show about 70% achieve mycologic cure and around 38% get complete cure in toenails. It works better than itraconazole in most studies. Yet relapse is common. Around 20%–25% of patients see the infection return within two years. Because of this, follow-up and preventive care are important. Doctors often use long-term strategies to keep nails healthy. Despite limits, terbinafine is still the gold standard for treating onychomycosis.
- Topical treatments are improving: Topical drugs are becoming more effective, though they are still weaker than oral options. Efinaconazole 10% used daily for 48 weeks shows a 15%–18% complete cure rate in trials. Tavaborole 5% achieves about 6.5%–9%. Ciclopirox 8% is lower, around 6%–9%. These treatments are best for mild or moderate cases. They are also used when oral drugs are unsafe. Doctors often combine topicals with debridement to improve results. While not as strong as oral terbinafine, topicals offer safer choices. They are gaining traction for patients who need long-term or low-risk care.
- Combination and maintenance strategies: Relapse remains a big issue in nail fungus care. To reduce this, doctors now use maintenance therapy. One method is adding a topical antifungal after oral terbinafine. For example, using a topical twice weekly after oral treatment cut recurrence from 76% down to 33% in a study. This approach gives longer-lasting results. Combination therapy also helps improve cure rates overall. Many clinics are now adopting this step to keep nails clear. Preventing relapse is becoming as important as achieving cure in the first place. This trend is reshaping long-term treatment strategies.
- Antifungal resistance and new pathogens: Resistance is an emerging challenge in treating nail fungus. A new species, Trichophyton indotineae, carries genetic changes that make terbinafine less effective. The first U.S. cases appeared in 2023, with updates from the CDC in 2024. This means doctors now focus more on lab testing and fungal species identification. When terbinafine fails, they consider alternative treatments. The rise of resistant infections is pushing research toward new drug options. It also highlights the need for smart prescribing. The trend shows how onychomycosis treatment is evolving beyond just one mainstay drug.
- Diagnostics before treatment: Guidelines stress confirming fungal infection before treatment begins. Simple tests like KOH, culture, or molecular methods help avoid misdiagnosis. This step matters because many nail problems mimic fungus. For oral terbinafine, baseline liver enzyme tests are also advised. Doctors also check for drug interactions, especially in patients with other health issues. These checks are now routine in many clinics. The goal is to make treatment safer and more effective. Confirming the diagnosis saves patients time and money. It also improves cure rates by making sure the right drug is chosen.
- Lasers and devices: Lasers and devices are getting attention, but approval is limited. Regulatory bodies have cleared them only for cosmetic nail improvement, not as cures. Evidence so far is mixed. Reviews, including Cochrane analyses, show low certainty. Some small trials suggest a benefit, but large trials are still missing. Many insurers still classify laser therapy as investigational or cosmetic. Patients often pay out of pocket for these sessions. Despite high interest, doctors view lasers as secondary options. They are not yet mainstream treatments. Until more strong data appear, drugs remain the primary therapy.
- Pediatrics and special populations: Treating children and special populations remains a challenge. No systemic antifungal is FDA-approved for children. Still, doctors sometimes use terbinafine or itraconazole off-label when needed. In patients with liver disease or many medications, oral antifungals can be risky. In these cases, topicals are safer choices. Doctors also recommend close monitoring by specialists. Safety is the top concern for these groups. Because of this, personalized care plans are more common. Topical therapy, combined with careful management, is shaping treatment approaches for patients with special health needs.
- Safety and monitoring: Safety plays a major role in treatment choice. Terbinafine can affect the liver, so baseline liver function tests are advised. Itraconazole carries a boxed warning for heart failure and has many drug interactions. These risks guide how doctors prescribe antifungals. Patients often undergo regular monitoring during therapy. These steps help catch side effects early. As a result, safety checks are now built into treatment plans. Doctors weigh risks against benefits before starting systemic therapy. This focus on patient safety is a driving trend in the market for nail fungus care.
- Adjunctive care: Adjunctive care is now standard practice in nail fungus management. Simple steps like nail trimming and debridement improve treatment success. They allow topical or oral drugs to penetrate better. Debridement also speeds up visible results, especially in thick or damaged nails. Many clinics now offer these services as part of treatment plans. They are safe, affordable, and widely accepted. The combination of procedural care with medication is proving more effective than drugs alone. This integration is making adjunctive care a key part of modern onychomycosis therapy.
- Teledermatology and adherence support: Sticking to treatment is one of the hardest parts of nail fungus care. Topical therapy often needs daily use for up to 48 weeks. Toenails may take 9–18 months to look fully clear. To help, clinics now use teledermatology and at-home support. Remote check-ins and digital reminders keep patients on track. This approach improves adherence and real-world outcomes. It also reduces the number of missed treatments. Patients benefit from easier follow-up without frequent clinic visits. These tools are becoming important in long-term care strategies. They bring technology into nail fungus management.
Use Cases
- Mild to Moderate Toenail Disease: For mild to moderate onychomycosis (≤50% of the nail, limited nails), topicals are the first choice. Commonly used drugs are efinaconazole 10% or tavaborole 5%, applied for 48 weeks. Regular debridement improves results. Expected cure rates vary: 15%–18% for efinaconazole and 6.5%–9% for tavaborole. Success rates are better if the infection is mild and patients stick to the regimen. The main reason for choosing topicals is safety. They avoid the liver and drug interaction risks seen with oral antifungals, making them suitable for many patients who want a low-risk treatment path.
- Moderate to Severe Toenail Disease: For more severe disease with nail matrix involvement or multiple nails affected, oral therapy is the standard. Oral terbinafine 250 mg daily for 12 weeks is the first-line option. Cure rates are significantly higher compared to topicals, with about 70% mycologic cure and 38% complete cure. Visible nail improvement is faster than with topical treatments. To prevent relapse, maintenance with twice-weekly topical antifungal is often added. This step reduces recurrence from 76% down to 33%. This combined strategy helps maintain long-term results and reduces the frustration of re-infection.
- Patients with Polypharmacy or Liver Risk: In patients with many medications or health concerns, treatment choices change. Topical antifungals are usually preferred to avoid drug interactions. If oral therapy is needed, doctors check baseline liver function tests (LFTs) before prescribing terbinafine. Itraconazole is avoided in patients with existing or potential heart failure due to safety concerns. Careful review of all current medicines is critical to reduce risks. Guidelines recommend balancing effectiveness with safety. For these patients, avoiding complications is just as important as clearing the infection. This group benefits from personalized treatment that reduces risks linked to systemic antifungals.
- Antifungal Resistance or Treatment Failure: When standard therapy fails, resistance should be suspected. The first step is to confirm the diagnosis with KOH, culture, or molecular tests. If terbinafine resistance is likely, species testing can guide the next steps. New resistant strains such as Trichophyton indotineae have been increasingly reported since 2023. In these cases, doctors may switch to itraconazole or other alternative regimens. The decision depends on both lab results and clinical progress. This approach ensures patients are not left with long-term infection. Addressing resistance early helps improve outcomes and reduces unnecessary exposure to ineffective treatments.
- Diabetes and High-Risk Foot Care: Onychomycosis is a bigger problem for people with diabetes or fragile feet. Thickened nails increase pressure on skin, which may cause breaks and bacterial infections. Because of this, early treatment is recommended. Oral terbinafine is often used if there are no health risks. Regular debridement and footwear hygiene reduce trauma. Relapse prevention is vital, since 20%–25% of cases recur within two years. Doctors often suggest topical prophylaxis after treatment. This strategy protects skin, reduces complications, and supports foot health in high-risk patients. Good management helps prevent serious infections and lowers the risk of ulcers.
- Cosmetic Nail Improvement: Some patients focus on nail appearance rather than infection clearance. In such cases, laser therapy is sometimes used. It can make the nail look clearer and thinner, improving cosmetic appeal. However, it is not FDA-approved for treating fungal infection itself. Evidence for laser effectiveness remains limited, with only a few high-quality studies available. Most insurers classify it as a cosmetic option, so it is not covered by health plans. Doctors may offer it as an adjunct treatment, especially when patients are infection-free but still want nails to look better.
- Pediatric and Adolescent Cases: Children and adolescents are a special group. No oral antifungals are officially FDA-approved for them. For mild cases, topicals are first-line. If the infection is widespread or persistent, doctors may carefully use oral terbinafine or itraconazole off-label, with close monitoring. The decision depends on the risk–benefit ratio and how well the child can follow treatment. Pediatric cases require extra care to ensure safety and long-term success. Doctors weigh the infection burden against potential drug risks before moving beyond topical options.
- Primary Care and Community Dermatology Protocols: Most patients are first seen in primary care or local dermatology clinics. A clear workflow improves results. Step one is confirming fungal infection to avoid treating look-alike nail problems. If terbinafine is chosen, doctors check liver tests and drug interactions first. Patients are told that nail recovery is slow, sometimes taking up to 18 months to look normal even if cured. Routine debridement, shoe and sock disinfection, and maintenance topical therapy after oral treatment are key parts of the protocol. Education and follow-up improve cure rates and reduce relapse.
Conclusion
The onychomycosis treatment market is set for steady growth, supported by an aging population, rising diabetes cases, and stronger healthcare focus on fungal infections. Oral terbinafine remains the gold standard, while improved topical drugs and combination therapies expand patient options. Resistance concerns and diagnostic advances are shaping treatment decisions, pushing demand for accurate testing and safer long-term care. North America leads due to advanced infrastructure, but awareness and adoption are growing worldwide. With affordability improving through generics and technology-driven care, the market shows stable opportunities. Innovation, patient safety, and relapse prevention will guide future strategies, ensuring consistent demand for effective nail fungus treatments.
View More
Fungal Staining Reagent Market || Medical Laser Market || Ophthalmic Lasers Market || Photodynamic Therapy Market || Cosmetic Surgery Market || Teledermatology Market || Dermatology Market
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



