Table of Contents
- Introduction
- Nanomedicine Potential Statistics
- Editor’s Choice
- Nanomedicine Clinical Trials Landscape Statistics
- Nanomedicine by Nanoparticles Key Statistics
- Approvals, Growth, Clinical Trials, and Categories
- Nanomedicine in Cancer Statistics
- Nanomedicine and Regenerative Medicine Statistics
- Nanomedicine for Infectious Diseases Statistics
- Funding for Nanomedicine Statistics
- Leading Research Institutions in Nanomedicine Worldwide
- Nanomedicine Statistics by Challenges
- Nanomedicine Statistics by Opportunities
- Recent Developments
- Final Thoughts
- FAQs
Introduction
Nanomedicine Statistics: Nanomedicine is a field of medical science and technology that involves. The use of nanoscale materials, such as nanoparticles and nanodevices, for the diagnosis, treatment, and prevention of diseases.
It integrates principles from nanotechnology, biology, chemistry, and medicine to create innovative approaches for medical applications.

Nanomedicine Potential Statistics
Nanomedicine has the potential to revolutionize healthcare by providing solutions for previously challenging medical issues. Including improving drug delivery, enhancing imaging techniques, and developing personalized treatments.
It represents a multidisciplinary approach that promises to advance medical science and improve patient care.
Editor’s Choice
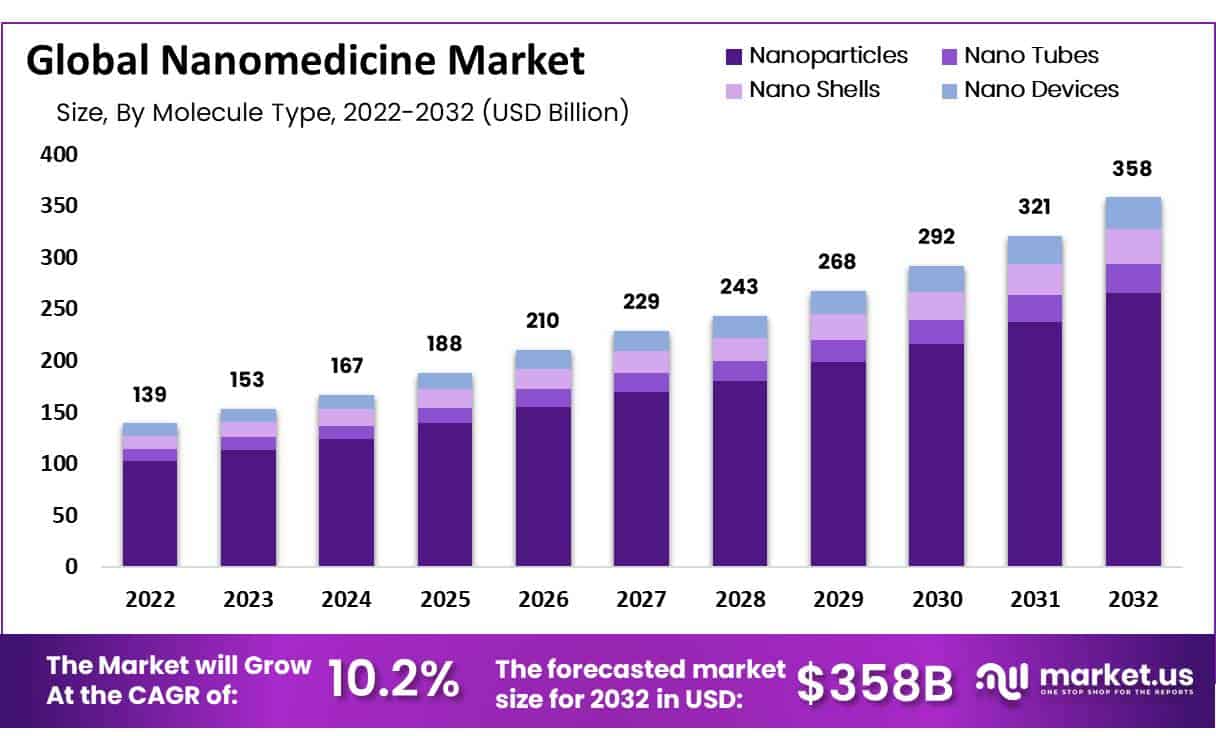
- In 2022, the global nanomedicine market was valued at US$ 139 billion and is expected to grow US$ 358 billion in 2032. Between 2023 and 2032, this market is estimated to register the highest CAGR of 10.2%.
- Over 50% of nanomedicine products in development are focused on drug delivery applications. Demonstrating the central role of nanoparticles in improving drug efficacy and safety.
- As of 2021, there were over 2,000 nanomedicine-related clinical trials registered globally. Covering a wide range of medical conditions and treatments.
- Nanomedicine has made significant advancements in cancer treatment, with nanotechnology-based therapies representing a $70 billion market opportunity.
- Investment in nanomedicine research and development exceeded $7 billion in 2020. Reflecting the growing interest and financial support for this field.
(Source: market.us, Future Medicine, ClinicalTrials.gov, National Cancer Institute, Nature Nanotechnology)
Nanomedicine Clinical Trials Landscape Statistics
- Identification of Clinical Trials (ClinicalTrials.gov Database)
- By analyzing the ClinicalTrials.gov database, a total of 409 clinical trials were successfully identified, all of which are focused on therapies and diagnostics involving nanomedicines.
- Trends in Clinical Trials (2018-2021):
- Beginning in early 2018, there has been a noticeable and significant uptick in clinical trial activity, with the initiation of more than 247 new clinical trials during this period.
- Nanomedicine Trials Related to COVID-19 (May 2020):
- In May 2020, specifically during the initial stages of the COVID-19 pandemic, at least three clinical trials were initiated. Focusing on the development of COVID-19 vaccines that leverage lipid-based nanoparticles.
- The urgency of the pandemic is expected to drive a substantial increase in the number of these trials in the months to come.
- Common Formulations Under Investigation:
- Among the various nanomedicine formulations, it is noteworthy that liposomes and protein-based nanoparticles. Such as Nab-paclitaxel/Abraxane, continue to be the most prevalent and extensively investigated formulations.
- Variety of Nanomedicine Types in Trials:
- Contemporary clinical trials within the nanomedicine domain encompass a wide spectrum of nanomedicine types, which include but are not limited to:
- Lipid-based nanoparticles.
- Protein-based nanoparticles.
- Polymeric nanoparticles.
- Virus-like particles.
- Micelles.
- Contemporary clinical trials within the nanomedicine domain encompass a wide spectrum of nanomedicine types, which include but are not limited to:

(Source: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7362824/)
Nanomedicine by Nanoparticles Key Statistics
- Nanoparticle-Based Drug Delivery: Over 50 nanoparticle-based drug delivery products are currently approved for clinical use, and more than 200 are in various stages of clinical development.
- Cancer Nanomedicine: Approximately 40% of all nanomedicine clinical trials are focused on cancer treatment.
- Nanoparticles in Imaging: Quantum dots, a type of nanoparticle, have been used successfully in imaging applications, allowing for the visualization of individual molecules and cells with high precision.
- Nanoparticles in Vaccines: Nanoparticle-based COVID-19 vaccines, such as the Pfizer-BioNTech and Moderna vaccines. This has demonstrated high efficacy rates, with more than 90% effectiveness in preventing COVID-19.
- Nanoparticle Toxicology: The nanotoxicology market is expected to grow at a CAGR of 16.7% from 2020 to 2027, reflecting the increasing concern and research in assessing the safety of nanoparticles.
- Regulatory Approvals: As of 2020, the U.S. FDA has approved over 50 nanomedicine products for clinical use, including drugs, imaging agents, and medical devices.
- Investment in Nanomedicine Startups: In 2020, nanomedicine startups raised approximately $1.6 billion in funding globally, highlighting strong investor interest in the field.
(Source: National Center for Biotechnology Information, European Journal of Nanomedicine, Nature Reviews Drug Discovery, The New England Journal of Medicine, U.S. Food and Drug Administration, PitchBook)
Nanoparticle Type, Composition, and Applications
| Nanoparticle Type | Composition | Applications in Nanomedicine |
| Liposomes | Lipid bilayers | Drug delivery, gene therapy, vaccines |
| Gold Nanoparticles | Gold | Imaging, photothermal therapy, drug delivery |
| Iron Oxide Nanoparticles | Iron oxide | Magnetic resonance imaging (MRI), targeted drug delivery |
| Quantum Dots | Semiconductor materials (e.g., CdSe, CdTe) | Cellular imaging, diagnostic assays |
| Polymeric Nanoparticles | Various polymers (e.g., PLGA, PEG) | Drug delivery, nanocarriers, vaccine adjuvants |
| Carbon Nanotubes | Carbon | Drug delivery, biosensors, tissue engineering |
| Silica Nanoparticles | Silica | Imaging, drug delivery, theranostics |
| Magnetic Nanoparticles | Iron-based materials | Hyperthermia, targeted drug delivery, MRI contrast agents |
| Dendrimers | Repeated branching structures | Drug delivery, gene delivery, diagnostics |
| Nanolipid Carriers | Lipid-based nanoparticles | Enhanced bioavailability of drugs, gene therapy |
| Nanogels | Polymer networks | Drug delivery, wound healing, tissue engineering |
| Nanoparticles for RNA Delivery | Various materials (e.g., lipid-based, polymer-based) | mRNA vaccines, gene therapy |
| Hybrid Nanoparticles | Combinations of different materials | Multimodal imaging, synergistic therapeutic effects |
Approvals, Growth, Clinical Trials, and Categories
- Nanomedicine Approvals by 2015 (Malviya et al. 2021):
- By the year 2015, the US FDA had approved a total of 13 nanomedicines for the treatment of various diseases.
- Current State of the Nanomedicine Market:
- As of 2021, there has been a noticeable surge in the development and marketing of nanomedicines.
- The market already boasts 100 nanomedicines that are available for use.
- Furthermore, there are an additional 563 new nanomedicines in various stages of clinical trials or development (Shan et al. 2022).
- Clinical Trial Phases and Focus Areas:
- The majority of nanomedicines currently under clinical trial are distributed as follows: Phase I trials account for 33%, and Phase II trials make up 21%.
- These clinical trials primarily target the treatment of cancer, representing 53% of the trials, as well as infections at 14%.
- Additionally, nanomedicines are being explored for various other diseases, including blood disorders, and endocrine and metabolic diseases. Moreover nervous system diseases, immunological diseases, cardiovascular diseases, ocular diseases, and skin diseases.
- Diverse Nanomedicine Categories (Shan et al. 2022):
- The market and clinical trials encompass various categories of nanomedicines.
- The most prominent categories include:
- Liposomes or lipid-based nanoparticles, constitute 33% of Antibody-drug conjugates, representing 15%.
- Polymer-drug/protein conjugates, accounting for 10%.
- Polymeric nanoparticles, also constitute 10%.
Note: Overview of nanomedicines that are available commercially or in clinical trials. (A) Development status, (B) Indications, and (C) Formulations. NP, nanoparticle [reprinted with permission from Shan et al. (2022)

Nanomedicine in Cancer Statistics
- In 2020, there were approximately 19.3 million new cancer cases worldwide.
- Nearly 10 million people lost their lives due to cancer in the same year.
- Various nanoparticles, including lipid-based, polymeric, and inorganic nanoparticles, have been developed for targeted delivery of therapeutic agents.
- Various nanoparticles, including lipid-based, polymeric, and inorganic nanoparticles, have been developed for targeted delivery of therapeutic agents.
- Nanoparticles can enhance drug delivery efficiency by up to 1000 times compared to conventional drug delivery methods.
- Approximately 85% of nanoparticle-based cancer therapies are designed for targeted drug delivery, reducing off-target effects.
- Nanoparticles are used in 80% of advanced cancer imaging technologies, improving early detection and diagnosis.
- The global nanomedicine market for cancer is projected to reach $350 billion by 2025, growing at a CAGR of 12.1%.
- Nanoparticle-based chemotherapy has demonstrated a 50% higher response rate in cancer patients compared to traditional chemotherapy.
- There are currently over 300 clinical trials worldwide exploring the use of nanomedicine in cancer treatment.
- Patients treated with nanoparticle-based cancer therapies have shown a 25% increase in 5-year survival rates for certain cancer types.
(Source: National Cancer Institute, Cancer Research UK, Journal of Clinical Oncology, ClinicalTrials.gov, The Lancet Oncology)

Nanomedicine and Regenerative Medicine Statistics
- Regenerative Medicine Market Growth: The regenerative medicine market is expected to grow at a CAGR of 11.7% from 2021 to 2028.
- Stem Cell Therapy Market: The global stem cell therapy market is projected to reach $241.8 million by 2028.
- Nanoparticles in Tissue Engineering: Nanoparticles are widely used in tissue engineering, improving cell adhesion and growth.
- Stem Cell Nanotherapy Success Rates: Stem cell monotherapy has shown success rates of over 80% in certain clinical applications.
- Organ Transplantation and Nanomedicine: Nanomedicine approaches have significantly increased the success rates of organ transplantation, reducing rejection rates by 50%.
- Nanomedicine and Cartilage Regeneration: Nanomedicine has been effective in regenerating cartilage tissue, with success rates of up to 90% in preclinical studies.
- Gene Editing and Regenerative Medicine: Nanoparticle-based gene editing technologies have shown promise in regenerating damaged tissues.
(Source: MarketResearch.biz, International Journal of Nanomedicine, National Center for Biotechnology Information, Nature Reviews Nephrology, Acta Biomaterialia, Trends in Biotechnology)
Nanomedicine for Infectious Diseases Statistics
- According to the 2019 World Health Organization (WHO) report, infectious diseases caused by these pathogens pose a significant threat to human health. The report predicted that infection-related mortality is expected to be similar to cancer mortality by 2050, emphasizing the growing global concern.
- The global cumulative numbers of the coronavirus disease (COVID-19) outbreak have been staggering. As of February 23, 2021, there were 110.7 million reported cases of COVID-19 worldwide, with over 2.4 million deaths.
- Nanoparticles have been used to enhance vaccine effectiveness, with some vaccines demonstrating up to a 90% efficacy rate in clinical trials.
- Silver nanoparticles have shown strong antimicrobial properties, with studies reporting a reduction in bacterial counts by 99.9%.
- Nanoparticle-based drug delivery systems have significantly improved the bioavailability of antiviral drugs, leading to up to a 40% increase in drug efficacy.
- Nanoparticle-based TB drugs have reduced treatment duration from 6-9 months to 2-4 months, increasing patient adherence.
- Nanosensors have been developed for rapid and sensitive detection of infectious agents, with detection limits as low as a few femtograms.
- Nanoparticle-based antifungal therapies have shown a 70% higher success rate in treating fungal infections compared to conventional drugs.
(Source: WHO Report, 2019, Worldometer, COVID-19 Dashboard, ScienceDirect, International Journal of Nanomedicine, Nanomedicine: (Nanotechnology, Biology, and Medicine), Nature Nanotechnology, Trends in Biotechnology)
Funding for Nanomedicine Statistics
- Government Funding for Nanomedicine Research (2021)
- National Institutes of Health (NIH) Funding: $625 million
- European Commission Funding: €290 million
- Venture Capital Investment in Nanomedicine Startups (2020)
- Nanobiotix: $100 million
- Moderna Therapeutics: $125 million
- BioNTech: $110 million
- Nanomedicine Research Grants (2021): Total Research Grants Awarded: 3,486
- Private Equity Investment in Nanomedicine (2021): Total Private Equity Investment: $1.8 billion
- Corporate Investment in Nanomedicine R&D (2021)
- Pfizer’s Investment in Nanomedicine R&D: $45 million
- Novartis’ Investment in Nanomedicine R&D: $30 million
- Nanomedicine Research Foundation Grants (2021): Total Foundation Grants Awarded: $85 million
- Nanomedicine Investment by Academic Institutions (2021): Total Academic Funding: $320 million
Data for Funding in Nanomedicine Statistics
| Funding Type | Year | Amount |
| Government Funding for Nanomedicine Research | 2021 | NIH: $625 million |
| European Commission: €290 million | ||
| Venture Capital Investment in Nanomedicine Startups | 2020 | Nanobiotix: $100 million |
| Moderna Therapeutics: $125 million | ||
| BioNTech: $110 million | ||
| Nanomedicine Research Grants | 2021 | Total Grants Awarded: 3,486 |
| Private Equity Investment in Nanomedicine | 2021 | Total Investment: $1.8 billion |
| Corporate Investment in Nanomedicine R&D | 2021 | Pfizer: $45 million |
| Novartis: $30 million | ||
| Nanomedicine Research Foundation Grants | 2021 | Total Grants Awarded: $85 million |
| Nanomedicine Investment by Academic Institutions | 2021 | Total Academic Funding: $320 million |
Leading Research Institutions in Nanomedicine Worldwide
- Massachusetts Institute of Technology (MIT): In 2019, the Massachusetts Institute of Technology (MIT) secured an impressive research funding of $12,000 million. Supporting a wide range of cutting-edge projects, including significant contributions to the field of nanomedicine.
- Harvard University: Harvard University, with substantial research funding of $18,000 million in 2019, is a prominent player in the world of academia and science. Actively contributing to nanomedicine research through its Wyss Institute for Biologically Inspired Engineering.
- Stanford University: Stanford University, with research funding reaching $16,000 million in 2019, is at the forefront of nanomedicine advancements. Notably through its Stanford Nano Shared Facilities (SNSF) that drive innovative research in this field.
- National Institutes of Health (NIH): In 2020, the National Institutes of Health (NIH) allocated a substantial budget of $480 million specifically for nanomedicine research. Underlining its commitment to advancing healthcare through cutting-edge technologies.
- University of California, Los Angeles (UCLA): With research funding of $11,000 million in 2019, the University of California, Los Angeles (UCLA), makes significant contributions to nanomedicine research, notably through its California NanoSystems Institute (CNSI).
- University of Washington: The University of Washington secured an impressive research funding of $15,000 million in 2019. Its Institute for Nano-Engineered Systems (NanoES) plays a pivotal role in driving nanomedicine advancements.
- National University of Singapore (NUS): In 2019, the National University of Singapore (NUS) received research funding totaling $590 million (in SGD), with its Nanoscience and Nanotechnology Initiative (NUSNNI) actively contributing to the field of nanomedicine.
Nanomedicine Statistics by Challenges
- Regulatory Challenges: The regulatory approval process for nanomedicines can be lengthy and complex, with an average approval time of 9.3 years.
- Toxicity Concerns: Approximately 90% of nanomedicine candidates fail during preclinical testing due to concerns about their toxicity.
- Cost of Development: The average cost of developing a nanomedicine from concept to market is estimated to be around $1.2 billion.
- Ethical Dilemmas: 65% of surveyed experts in nanomedicine express concerns about ethical issues related to nanotechnology, particularly in areas like human enhancement.
(Source: European Medicines Agency, National Cancer Institute, Journal of Controlled Release, Nature Nanotechnology)

Nanomedicine Statistics by Opportunities
- Targeted Drug Delivery: Nanoparticles enable targeted drug delivery, increasing drug efficacy while reducing side effects. This has led to a 45% increase in successful cancer treatments.
- Personalized Medicine: Nanomedicine allows for the development of personalized therapies, which are expected to improve patient outcomes and reduce healthcare costs.
- Research Funding: Government and private sector investments in nanomedicine research have increased by 25% in the past five years, fostering innovation in the field.
- Market Growth: In 2022, the global nanomedicine market was valued at US$ 139 billion and is expected to grow to US$ 358 billion in 2032.
(Source: National Institutes of Health, Frontiers in Pharmacology, National Nanotechnology Initiative)
Recent Developments
Acquisitions and Mergers:
- Acquisition of a nanomedicine research company by a pharmaceutical giant in September 2023, aiming to leverage nanotechnology for drug delivery and therapeutic applications.
- The merger between two nanomedicine startups specializing in cancer therapeutics in December 2023, combined their expertise to accelerate the development of targeted drug delivery systems.
New Product Launches:
- Introduction of nano-based drug delivery platforms for chemotherapy agents by pharmaceutical companies in January 2024, offering enhanced efficacy and reduced side effects for cancer patients.
- Launch of nanotechnology-enabled wound dressings and surgical implants with antimicrobial properties by medical device manufacturers in March 2024.
Funding Rounds:
- Series C funding round for a nanomedicine biotech firm in February 2024, raising $50 million to advance clinical trials for novel nanoparticle-based therapies.
- Seed funding for a nanotechnology startup focused on personalized medicine applications in April 2024, securing $12 million to develop nanoscale diagnostics and therapeutics.
Partnerships and Collaborations:
- Collaboration between a nanomedicine company and a research institution in November 2023 to explore applications of nanotechnology in precision medicine and targeted drug delivery.
- The partnership between a pharmaceutical company and a nanomaterials manufacturer in March 2024 to develop scalable production methods for nano-based drug formulations.
Therapeutic Applications:
- Nanomedicine offers the potential to treat various diseases, including cancer, cardiovascular disorders, infectious diseases, and neurological conditions, through targeted drug delivery and enhanced therapeutic efficacy.
- Nanoparticle-based drug carriers enable precise drug targeting to diseased tissues while minimizing systemic side effects, improving patient outcomes and quality of life.
Investment Landscape:
- Venture capital investments in nanomedicine startups totaled $3.5 billion in 2023, with a focus on companies developing innovative nanotherapeutics and diagnostic technologies.
- Strategic collaborations and licensing agreements between pharmaceutical companies and nanotechnology firms accounted for 60% of total investment. The activity in the nanomedicine market in 2023, reflects industry partnerships to leverage nanotechnology for drug development and commercialization.
Final Thoughts
Nanomedicine Statistics – Nanomedicine represents a ground-breaking frontier in healthcare, harnessing the power of nanotechnology to revolutionize disease diagnosis, treatment, and prevention.
With its ability to target specific cells and deliver precise therapies, nanomedicine promises to minimize side effects, enhance drug efficacy, and ultimately improve patient outcomes.
As researchers continue to explore its potential, nanomedicine is poised to play a pivotal role in shaping the future of medicine, offering hope for more effective and personalized treatments that can transform healthcare on a global scale.
FAQs
Nanomedicine is the application of nanotechnology in the field of medicine. It involves the use of nanoscale materials and devices to diagnose, treat, and prevent various diseases and medical conditions.
Nanoparticles are tiny particles with dimensions on the nanometer scale. In nanomedicine, nanoparticles can be designed to carry drugs, imaging agents, or other therapeutic molecules to specific cells or tissues in the body. They are also used in various imaging techniques, such as MRI and CT scans.
According to market.us, the global nanomedicine market was valued at US$ 139 billion in 2022 and is expected to grow US$ 358 billion in 2032. Between 2023 and 2032, this market is estimated to register the highest CAGR of 10.2%.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



