Table of Contents
Introduction
Global Mycoplasma Testing Market size is expected to be worth around USD 2338 Million by 2032 from USD 860 Million in 2023, growing at a CAGR of 12.11% during the forecast period from 2024 to 2032.
The Mycoplasma Testing Market is experiencing significant growth due to the rising incidence of mycoplasma infections and the increasing demand for rapid and accurate testing methods. Mycoplasmas are a type of bacteria without a cell wall, making them resistant to many common antibiotics, which complicates treatment. The need for precise diagnosis has led to advancements in molecular testing techniques, such as PCR, which offer higher sensitivity and specificity compared to traditional methods.
Several factors are driving the growth of the Mycoplasma Testing Market. The increasing prevalence of respiratory infections caused by Mycoplasma pneumoniae, particularly in crowded environments like schools and nursing homes, has heightened the need for effective diagnostic tools. Furthermore, the rise of antibiotic-resistant strains necessitates robust testing to guide appropriate treatment strategies.
However, the market faces challenges, including the complexity of accurately detecting mycoplasmas due to their unique characteristics and the high costs associated with advanced testing technologies. The development of rapid, cost-effective testing methods that can differentiate between viable and nonviable mycoplasma is crucial to overcoming these obstacles.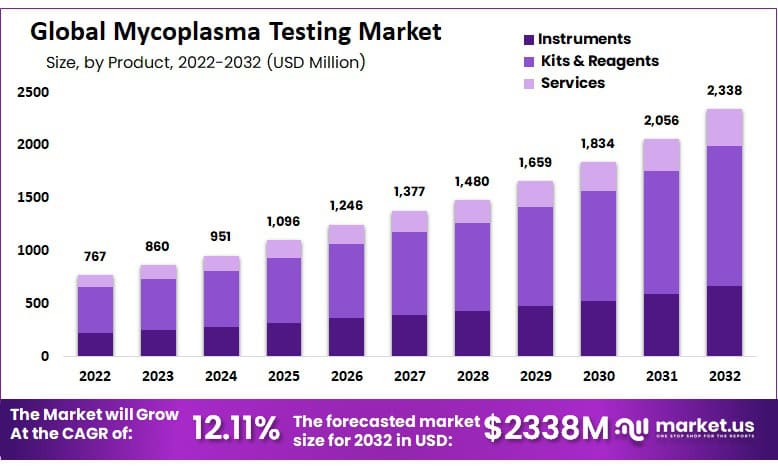
Recent developments in the market highlight the industry’s focus on innovation and collaboration. Leading companies are investing in research to improve the accuracy and efficiency of mycoplasma detection. Advances in nucleic acid-based testing methods and the integration of automated systems are helping to streamline the testing process, making it more accessible and reliable for clinical and research applications.
Key Takeaways
- Market Size: Mycoplasma Testing Market size is expected to be worth around USD 2338 Million by 2032 from USD 860 Million in 2023.
- Market Growth: The market growing at a CAGR of 12.11% during the forecast period from 2024 to 2032.
- By Product Analysis: The kit & reagents segment dominated with a market share of 55.6 % and a projected CAGR of 12.11% in 2022.
- By Technique Analysis: With a revenue share of 36.1% in 2022, The PCR segment dominated the mycoplasma testing market and is projected to grow fastest.
- By End User Analysis: Among these end-users, the pharmaceutical and biotechnology segment is estimated to be the most lucrative segment in the global mycoplasma testing market, with the largest revenue share of 36.8%.
- By Regional Analysis: In 2022, North America held a 40.7% revenue share, dominating the market. Europe is the second-largest market for mycoplasma testing, driven by the growing biopharmaceutical industry in the region.
- Technology Advancements: Molecular testing techniques, such as PCR, are leading the market due to their high sensitivity and specificity compared to traditional methods.
- Antibiotic Resistance: The rise in antibiotic-resistant mycoplasma strains is driving the need for robust testing methods to guide effective treatment strategies.
- Application Areas: The market sees high demand in environments prone to infections, such as schools and nursing homes, due to the commonality of respiratory infections in these settings.
- Challenges: Key challenges include the complexity of accurately detecting mycoplasmas and the high costs associated with advanced testing technologies.
- Industry Innovation: Recent developments focus on nucleic acid-based testing methods and automated systems, enhancing the accuracy and efficiency of mycoplasma detection.
Mycoplasma Testing Statistics
- PCR Test Duration: Results from Mycoplasma PCR testing can be obtained within 2-3 days.
- Culture Duration: Traditional Mycoplasma culture tests take 10-28 days to yield results.
- Species Detection: PCR testing can detect over 90 species of Mycoplasma, Acholeplasma, and Spiroplasma.
- Prevalence: Mycoplasma pneumoniae accounts for 10-30% of community-acquired pneumonia cases.
- Mycoplasma Genitalium Resistance: Up to 80% of Mycoplasma genitalium infections show resistance to macrolides.
- Testing Stability: Samples for Mycoplasma testing remain stable for 48 hours at 4°C or up to 2 months when frozen at -70°C..
- Sample Volume Requirement: Optimal fluid sample volume for PCR testing is 0.2-1 mL, and tissue volume is 0.3-1.0 cm³.
- Ureaplasma Detection: PCR testing can identify Ureaplasma species, including U. urealyticum and U. parvum.
- Test Sensitivity: Mycoplasma PCR testing uses genus-specific primers and species-specific FRET probes for high sensitivity.
- Clinical Relevance: Mycoplasma and Ureaplasma are responsible for 20-50% of all cases of non-gonococcal urethritis.
- Specimen Types: Acceptable specimens include fresh frozen tissue, cerebrospinal fluid, pleural fluid, and joint fluid.
- Diagnostic Accuracy: Mycoplasma PCR provides higher accuracy compared to traditional methods due to its ability to detect fastidious organisms that do not grow in culture.
Mycoplasma Testing Testing Type Analysis
- Cell Line Testing: Cell line testing for Mycoplasma contamination is crucial in research and biotechnology. PCR-based methods are commonly used, allowing detection of over 90 Mycoplasma species within 2-3 days. Regular screening is recommended before cell banking and cryopreservation to avoid contamination that can compromise experimental results and therapeutic production.
- Virus Testing: Virus testing in the context of Mycoplasma involves ensuring that viral cultures are free from Mycoplasma contamination, which can interfere with viral replication studies. PCR testing is the preferred method due to its sensitivity and speed, detecting contamination that traditional methods might miss, ensuring the integrity of viral production and research.
- End of Production Cells Testing: End of production cells testing is critical in biopharmaceutical manufacturing to ensure that final cell products are free from Mycoplasma contamination. This testing typically involves PCR methods, which provide rapid and reliable results, ensuring that the final product meets safety standards before release.
Emerging Trends
- Rise in Molecular Testing: Molecular testing techniques, particularly PCR and NAATs, are gaining traction due to their high sensitivity and specificity in detecting Mycoplasma pneumoniae infections, making them the preferred choice for diagnostics.
- Multi-Pathogen Testing: There is an increasing trend towards multi-pathogen testing, which allows for the simultaneous detection of Mycoplasma along with other respiratory pathogens, improving diagnostic efficiency.
- Antibiotic Resistance Monitoring: The growing issue of macrolide-resistant Mycoplasma strains is driving the demand for testing methods that can identify antibiotic susceptibilities, helping to guide appropriate treatment.
- Increased Testing in Crowded Environments: There is a heightened focus on testing in environments prone to outbreaks, such as schools and nursing homes, due to the commonality of Mycoplasma infections in these settings.
- Automation in Testing: The integration of automated systems in mycoplasma testing is improving throughput and reducing manual errors, making the process more efficient and reliable.
- Use of Alternative Methods: While culture methods are time-consuming, there is a shift towards using alternative techniques like nucleic acid-based assays that provide faster results without compromising accuracy.
- Global Surveillance Efforts: Enhanced global surveillance and reporting systems are being implemented to track Mycoplasma infections and resistance patterns, aiding in the development of better control strategies.
Use Cases
- Clinical Diagnostics: Mycoplasma testing is critical in diagnosing infections caused by Mycoplasma pneumoniae, a common cause of respiratory infections like “walking pneumonia.” Molecular methods such as PCR provide accurate results to guide treatment.
- Respiratory Infection Monitoring: Testing is essential for monitoring outbreaks of respiratory infections in crowded settings such as schools and nursing homes, helping to control the spread and implement timely interventions.
- Antibiotic Resistance Management: Mycoplasma testing helps identify antibiotic-resistant strains, enabling healthcare providers to select appropriate treatments and minimize the use of ineffective antibiotics, which is crucial for managing resistance.
- Public Health Surveillance: Public health laboratories use mycoplasma testing to track infection trends and inform public health strategies, particularly during periods of increased respiratory illness.
- Sexually Transmitted Infections: Mycoplasma genitalium is another pathogen detected through specialized tests, which is important for diagnosing and treating infections that can lead to reproductive health issues.
- Research and Development: Mycoplasma testing is used in R&D to evaluate new diagnostic tools and therapeutic approaches, contributing to advancements in infection control and treatment.
- Hospital Infection Control: Hospitals utilize mycoplasma testing to prevent and control hospital-acquired infections, ensuring patient safety and maintaining healthcare quality.
- Biopharmaceutical Production: In the biopharmaceutical industry, mycoplasma testing ensures that cell cultures used in the production of biologics are free from contamination, safeguarding product safety and efficacy.
Conclusion
The Mycoplasma Testing Market is witnessing strong growth due to the rising incidence of mycoplasma infections and the increasing need for rapid and accurate diagnostics. PCR-based methods dominate the market, driven by their high sensitivity and specificity, particularly in detecting antibiotic-resistant strains. However, challenges like high costs and complexity remain. Advances in molecular testing and automation are making diagnostics more efficient, while multi-pathogen testing is emerging as a key trend. The market’s expansion is supported by growing demand in clinical diagnostics, public health surveillance, and biopharmaceutical production, emphasizing the importance of accurate and timely detection in controlling infections and ensuring product safety.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



