Table of Contents
Overview
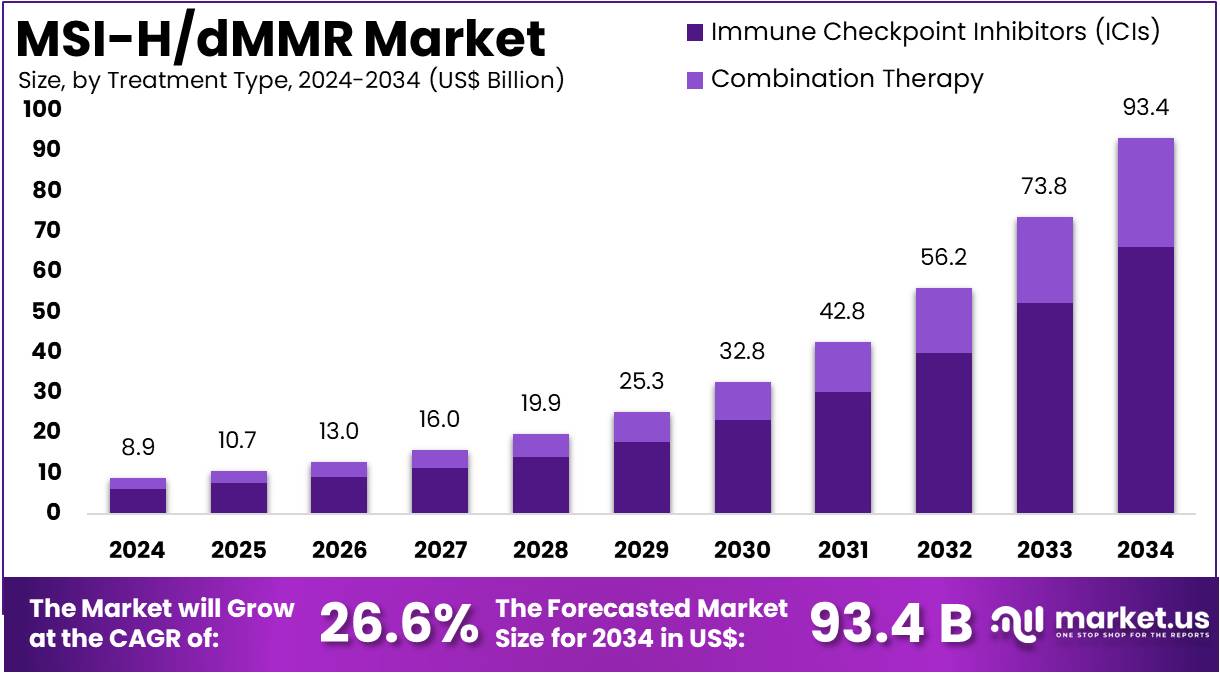
New York, NY – July 28, 2025 : The global MSI-H/dMMR market is projected to grow significantly. It is expected to reach around US$ 93.4 billion by 2034, up from US$ 8.9 billion in 2024. This reflects a strong CAGR of 26.6% during the forecast period from 2025 to 2034. North America currently leads the market with a 53.1% share, valued at US$ 4.7 billion. The region benefits from advanced healthcare infrastructure and high investment in precision oncology. The increasing adoption of targeted therapies further supports the region’s dominance in the global market.
One of the key drivers of market growth is the rising prevalence of MSI-H/dMMR cancers. These genetic mutations are common in colorectal, endometrial, and gastric cancers. They serve as crucial biomarkers for targeted and personalized treatments. This creates opportunities for drug developers and diagnostic companies. As more oncologists recognize the importance of MSI-H/dMMR status in treatment planning, demand for testing and targeted therapies is increasing. This growing awareness is expected to drive diagnostic innovation and patient screening rates worldwide.
Immunotherapies, especially immune checkpoint inhibitors, are accelerating market expansion. These therapies show strong efficacy in MSI-H/dMMR cancer patients, improving survival and treatment response. Drugs like pembrolizumab and nivolumab are now widely used for these indications. The success of such treatments is encouraging more research into novel immunotherapies. This trend is expected to expand the treatment landscape and offer hope to patients who previously had limited options. As a result, the market is seeing rapid innovation and product pipeline growth in this therapeutic segment.
However, some challenges may slow market growth. High costs of immunotherapies and companion diagnostics remain a barrier for widespread adoption. Access to specialized testing is still limited in some regions, affecting early detection. Regulatory complexity can also delay approvals of new treatments. Despite these issues, the market continues to attract investment. Pharmaceutical companies are working to reduce costs, improve testing availability, and overcome regulatory obstacles. Addressing these challenges will be key to unlocking the full potential of this fast-growing market.
On a positive note, ongoing research and clinical trials are opening new opportunities. Companies are exploring advanced biomarkers and combination therapies for better patient outcomes. Trials such as CheckMate-142 have shown promising results, supporting new approvals. For instance, the PMDA approved nivolumab in 2020 for MSI-H/dMMR colorectal cancer based on this trial. As global clinical efforts expand, more effective treatment options are expected to emerge. These developments are likely to strengthen market confidence and improve access to life-saving therapies for MSI-H/dMMR cancer patients.
Key Takeaways
- Market Growth Projection : An industry expert revealed that the MSI-H/dMMR market is set to surge from USD 8.8 billion in 2024 to USD 97.3 billion by 2034.
- Dominance of Immune Checkpoint Inhibitors : In 2024, immune checkpoint inhibitors led the market, contributing 71.0% of total revenue, driven by their growing clinical success and usage.
- Prevalence in Early-Stage Diagnosis : Early-stage cancer cases, particularly Stage I and II, accounted for 58.4% of global market revenue in 2024, indicating improved detection practices.
- Endometrial Cancer Segment Leadership : Endometrial cancer emerged as the largest application area in 2024, capturing 24.1% of the market, reflecting rising screening and diagnosis rates.
- Hospitals as Primary Treatment Centers : Hospitals dominated the market in 2024 with a 54.0% revenue share, underlining their role in delivering advanced MSI-H/dMMR-related therapies and care.
- North America’s Market Leadership : North America secured over 53.1% of global market revenue in 2024, backed by strong healthcare infrastructure and early adoption of targeted therapies.
Regional Analysis
In 2024, North America led the MSI-H/dMMR market, holding over 53.1% of the global share with a value of US$ 4.7 billion. The region’s dominance is driven by its advanced healthcare infrastructure, especially in the United States. Access to modern diagnostics and therapies supports the rapid use of treatments like immune checkpoint inhibitors. These therapies are commonly used for MSI-H/dMMR-positive cancers. The early adoption of such technologies is helping the region maintain its leading position in the global market.
North America also benefits from a strong pharmaceutical industry and regulatory support. Major drug companies in the region are pushing innovation and securing faster approvals for MSI-H/dMMR-targeted therapies. The FDA plays a key role in speeding up these approvals. Additionally, growing awareness of personalized cancer care is driving demand. More patients and doctors are seeking tailored treatment options, further boosting the market. As a result, North America remains a key growth area for MSI-H/dMMR therapies worldwide.
Segmentation Analysis
Treatment Type Analysis
The MSI-H/dMMR market is segmented by treatment type into immune checkpoint inhibitors (ICIs) and combination therapy. In 2024, ICIs held a dominant market share of 71.0%. ICIs, like pembrolizumab and nivolumab, are highly effective in treating MSI-H/dMMR cancers. These cancers have numerous mutations, making them more visible to the immune system. However, they use checkpoint proteins like PD-1 to avoid detection. ICIs block these proteins and help the immune system recognize and destroy cancer cells, especially in colorectal and endometrial cancers.
Stage Analysis
The market is also segmented by cancer stages: early stages (Stage I and II) and advanced stages (Stage III and IV). In 2024, early stages held 58.4% of the global market share. Early-stage MSI-H/dMMR cancers are treated with focused diagnostic and therapeutic strategies. These tumors are commonly found in colorectal, gastric, and endometrial cancers. Early detection improves treatment response. Studies show that about 5% to 15% of colorectal cancers are MSI-H/dMMR, making early-stage treatment a key area of growth in this market.
Indication Analysis
By indication, the market includes colorectal, gastric, endometrial, small intestine, cervical cancer, and others. Endometrial cancer led the market with a 24.1% share in 2024. MSI-H/dMMR markers are seen in 20%–30% of endometrial cancer cases. These tumors have a high mutational burden due to defective DNA repair. This makes them more likely to respond to immunotherapy. Their strong immunogenic profile makes endometrial cancer a leading focus in the development of precision oncology treatments and clinical trials involving immune checkpoint inhibitors.
End-User Analysis
The market is divided by end-user into hospitals, oncology clinics, research institutions, and homecare settings. In 2024, hospitals held the largest market share at 54.0%. Hospitals provide advanced treatment options and infrastructure needed for complex immunotherapy. Many MSI-H/dMMR cancers, like colorectal and gastric cancers, are diagnosed at late stages. These cases require aggressive treatment and ongoing monitoring. Hospitals are best equipped to handle therapies like ICIs, which are often delivered by infusion and require specialized care, making them central to MSI-H/dMMR cancer management.
Key Segments Analysis
By Treatment Type
- Immune Checkpoint Inhibitors (ICIs)
- Pembrolizumab (Keytruda)
- Nivolumab (Opdivo)
- Dostarlimab (Jemperli)
- Ipilimumab (Yervoy)
- Combination Therapy
- o ICI+Chemotherapy
- o Others
By Stage
- Early Stages (Stage I and II)
- Advanced Stages (Stage III and IV)
By Indication
- Endometrial
- Gastric
- Colorectal Cancer
- Small Intestine Cancer
- Cervical Cancer
- Others
By End User
- Hospitals
- Oncology Clinics
- Research Institutions
- Homecare
Key Players Analysis
The MSI-H/dMMR market is currently dominated by major pharmaceutical players like Merck, Bristol-Myers Squibb, and Roche. These companies are leveraging their immune checkpoint inhibitors to treat MSI-H/dMMR cancers effectively. Their strong R&D capabilities and extensive clinical networks give them a competitive edge. However, as the market grows, new players are entering the space. This is driving innovation and increasing treatment options for patients. The presence of big pharma ensures stability, but also creates high entry barriers for smaller companies.
At the same time, the competitive landscape is evolving. Smaller biotech firms are introducing novel therapies like CAR-T cell therapy, neoantigen vaccines, and oncolytic viruses. These treatments offer potential breakthroughs for resistant or advanced MSI-H/dMMR cancers. Ongoing clinical trials are expanding the indications for these therapies. As a result, the market is shifting towards combination therapies and personalized treatments. This trend reflects the growing need for targeted solutions to address unmet medical needs in the MSI-H/dMMR segment.
Leading Key Players in the MSI-H/dMMR Market
- Pfizer Inc.
- Merck & Co.
- AstraZeneca
- F. Hoffmann-La Roche Ltd
- Bristol-Myers Squibb Company
- Eli Lilly and Company
- GSK plc.
- BeiGene LTD.
- Summit Therapeutics Inc.
- Sanofi
- Novartis AG
- AbbVie Inc.
- Amgen Inc.
- Johnson & Johnson Services, Inc.
Emerging Trends
1. Expansion of Immunotherapy Treatments
Immunotherapy is changing the way doctors treat MSI-H/dMMR cancers. Immune checkpoint inhibitors, a type of immunotherapy, help the immune system find and destroy cancer cells. These drugs were first used mainly for colorectal cancer. Now, they are being used for other cancers like endometrial, gastric, and prostate cancer. This trend is growing fast. As more research proves their success, these treatments are becoming more common. Patients with MSI-H/dMMR markers now have better options and better chances of survival. With wider use across cancer types, immunotherapy is becoming a key part of personalized cancer care.
2. Combination Therapies Are Gaining Interest
Many doctors now believe one drug is not always enough. That’s why combination therapies are becoming popular. This involves using immunotherapy along with chemotherapy, radiation, or targeted drugs. These combinations can improve how well patients respond to treatment. They are especially helpful for patients who don’t do well with single-drug therapies. Research is ongoing to find the best mixes. Early results look promising. As more studies are published, combination treatments may become the new standard. This trend could offer longer survival and better quality of life for cancer patients with MSI-H/dMMR markers.
3. Liquid Biopsies for Easier Diagnosis
Traditional biopsies often require surgery or tissue removal. But now, liquid biopsies are becoming a popular choice. They use a simple blood sample to detect cancer markers like MSI-H or dMMR. This method is faster and less painful for patients. It also allows doctors to check for cancer more often during treatment. Liquid biopsies are being tested in clinical settings and are showing good results. As the technology improves, it may replace older methods. This trend is helping doctors catch cancers earlier and make better treatment decisions based on real-time data.
4. Earlier-Stage Treatment Adoption
MSI-H/dMMR treatments were first used for advanced cancers. Now, they are being tested in early-stage cancers too. Treating cancer earlier can stop it from spreading or returning. Doctors are trying immunotherapy before surgery or right after, during the recovery stage. This can improve the patient’s chances of staying cancer-free. Clinical trials are showing that early use of these treatments works well. As a result, guidelines may change soon to include them at earlier stages. This shift marks a big step toward proactive, rather than reactive, cancer care.
5. Personalized Medicine Is Growing
Every patient is different. Personalized medicine uses genetic testing to find the right treatment for each person. MSI-H/dMMR cancers respond well to certain drugs. By checking a patient’s genetic profile, doctors can see if these drugs will work. This avoids giving treatments that may not help. Personalized medicine reduces side effects and improves results. It also helps doctors track how the cancer responds and make changes quickly. As genetic testing becomes more available, personalized treatment will become the norm. This trend ensures patients receive the best care based on their unique biology.
6. Global Clinical Trials on the Rise
More MSI-H/dMMR therapies are being tested worldwide. Clinical trials are no longer limited to the U.S. and Europe. Countries in Asia, Latin America, and the Middle East are joining in. These trials bring new hope to patients in places where treatments were once limited. They also help gather data from different ethnic and genetic groups. This makes treatments more effective for more people. With growing global participation, results from trials are more reliable and inclusive. The trend of international trials is helping speed up the approval of new drugs for MSI-H/dMMR cancers.
Use Cases
1. Treating Colorectal Cancer
MSI-H/dMMR testing plays a key role in treating colorectal cancer. Doctors use this test to find out if a patient’s tumor has these mutations. If it does, the patient may respond better to immunotherapy instead of regular chemotherapy. Immunotherapy helps the immune system recognize and attack cancer cells. This makes treatment more targeted and effective. MSI-H/dMMR status helps guide doctors in making better treatment decisions. It also helps avoid unnecessary treatments that may not work. As a result, patients with MSI-H/dMMR colorectal cancer often have better outcomes and fewer side effects when treated with immune-based therapies.
2. Helping Patients with Endometrial and Gastric Cancers
MSI-H/dMMR testing isn’t just for colorectal cancer. It is also helpful for patients with endometrial (uterine) and gastric (stomach) cancers. These cancers can also carry MSI-H/dMMR mutations. Testing these tumors helps doctors decide if the patient is likely to respond to immunotherapy. This is especially useful when other treatments have failed. Immunotherapy can offer better outcomes for these patients. It also helps reduce side effects compared to chemotherapy. By using MSI-H/dMMR testing early, doctors can personalize the treatment plan. This makes care more effective and focused on what works best for the patient.
3. Genetic Screening for Hereditary Cancer
MSI-H/dMMR testing helps identify people with Lynch syndrome. This is a hereditary condition that raises the risk of several cancers, like colon, uterine, and stomach cancer. When someone tests positive, their family members can also get tested. If they also have the gene mutation, they can start early screenings or take preventive steps. This might include lifestyle changes, regular check-ups, or even preventive surgery. Early detection can save lives. MSI-H/dMMR testing is an important tool for families with a history of cancer. It supports both treatment and prevention efforts by guiding high-risk people toward timely care.
4. Evaluating Treatment Resistance
Sometimes, cancer stops responding to standard treatments. In such cases, MSI-H/dMMR testing helps doctors re-evaluate the tumor. It can show if the cancer has become more sensitive to newer drugs, such as immune checkpoint inhibitors. These drugs work by helping the immune system recognize and attack cancer cells. Testing for MSI-H/dMMR at this stage gives doctors a clearer picture of what treatment might work next. It helps avoid wasting time on therapies that may not be effective. This testing can improve outcomes for patients by guiding timely shifts to newer, better-targeted treatment options.
5. Monitoring Cancer Recurrence
MSI-H/dMMR testing can also be used to monitor patients after treatment. Doctors may include this test in follow-up care plans to watch for signs of cancer coming back. Detecting recurrence early gives doctors a chance to act quickly. This can lead to better chances of controlling the disease. Regular MSI-H/dMMR testing helps personalize the surveillance strategy for each patient. It provides valuable insights into how the disease behaves over time. With early detection, treatment can start sooner, improving the patient’s long-term outcome and quality of life.
6. Research and Drug Development
Pharmaceutical companies rely on MSI-H/dMMR testing during drug development. These markers help researchers find the right group of patients for clinical trials. For example, new immunotherapy drugs are often tested on patients with MSI-H/dMMR tumors. This increases the chances of seeing positive results during trials. It also helps speed up drug approval. Targeting these mutations allows companies to design more effective and personalized treatments. The use of MSI-H/dMMR in research helps reduce costs and improve success rates. It plays a vital role in developing the next generation of cancer therapies.
Conclusion
In conclusion, the MSI-H/dMMR market is growing quickly due to rising cancer cases, better diagnostics, and the success of immunotherapies. With strong support from hospitals, researchers, and drug makers, this market is set for major growth in the coming years. While there are challenges like high treatment costs and limited access in some regions, ongoing clinical trials and global research efforts are creating new opportunities. Personalized medicine, earlier diagnosis, and new therapy options are making cancer care more effective for patients with MSI-H/dMMR mutations. As awareness and testing improve worldwide, the market is likely to continue expanding and changing the future of cancer treatment.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



