Table of Contents
Introduction
Global Monoclonal Antibodies Market size is expected to be worth around USD 588.0 Billion by 2032 from USD 221.6 Billion in 2023, growing at a CAGR of 11.80% during the forecast period from 2024 to 2032.
Monoclonal antibodies (mAbs) represent a critical segment of immunotherapy, distinguished by their ability to bind specifically to targeted cells. These proteins, all derived from a single B-cell clone, have the unique capability to recognize and attach to distinct antigen sites. This specificity makes mAbs increasingly valuable for both diagnostic and therapeutic applications across a wide range of diseases, including cancer, cardiovascular disorders, autoimmune diseases, blood disorders, and infectious diseases.
Monoclonal antibodies are classified based on their origin into four types murine, chimeric, humanized, and human. Murine antibodies are entirely derived from mice, making them the least compatible with the human immune system. Chimeric antibodies blend murine and human components to reduce immunogenicity, while humanized antibodies are primarily human except for minor murine elements to enhance target specificity. Human antibodies are entirely derived from human protein sequences, offering the highest compatibility and lowest risk of immune rejection.
The commercial journey of monoclonal antibodies began in 1986 with the approval of the first therapeutic mAb. The market has since expanded robustly. By November 2014, forty-seven mAb products had received approval in the US and Europe, with several more gaining approval in other global markets. The approval rate of approximately four new products per year suggested that by 2020, around 70 monoclonal antibody products would be available, with combined global sales nearing $125 billion. This significant growth underscores the impact of mAbs in transforming treatment paradigms across various medical fields.
This analysis reflects the expansive role and economic impact of monoclonal antibodies within the pharmaceutical industry, highlighting their evolution from novel therapies to mainstream treatment options. The ongoing development and approval of new mAb products continue to drive substantial revenue growth and therapeutic advancements in healthcare.
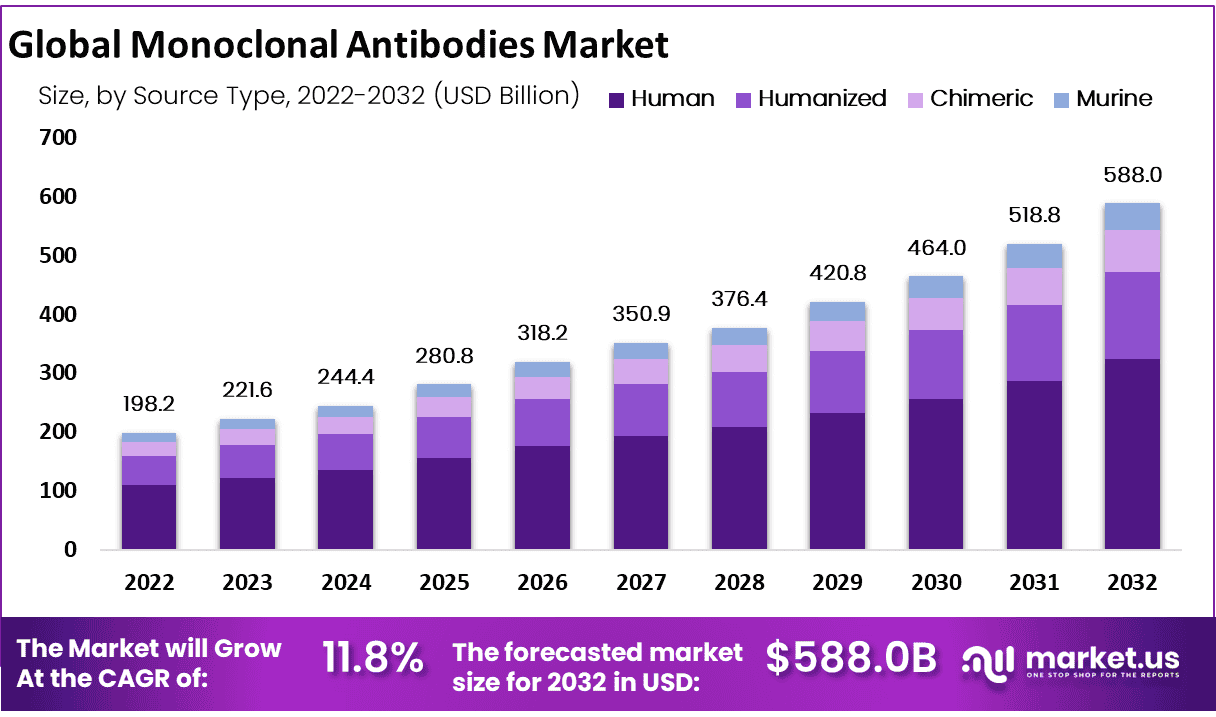
Key Takeaways
- Market Size: Global Monoclonal Antibodies Market size is expected to be worth around USD 588.0 Billion by 2032 from USD 221.6 Billion in 2023.
- Market Growth: The market growing at a CAGR of 11.80% during the forecast period from 2024 to 2032.
- Source Type Analysis: The human segment accounted for the largest market revenue share of 55.2% during the forecast period.
- Disease Indication Analysis: The Cancer Segment Accounted for the Highest Revenue Share of the Market.
- Distribution Channel Analysis: The hospital pharmacy segment dominated for the highest market revenue share of 43.0% in 2022
- Regional Analysis: North America recorded the highest market revenue share of 46.5% during the forecast period.
Monoclonal Antibodies Statistics
Types of Monoclonal Antibodies:
- Chimeric Antibodies: Approximately 65% human, these antibodies combine murine variable regions with human constant regions to reduce immune responses against them.
- Humanized Antibodies: About 95% human, these are designed by grafting murine hypervariable regions into human antibodies to significantly decrease their immunogenicity.
Clinical Trials and Effectiveness:
- Lecanemab: In phase 3 trials, it showed a 27% slower cognitive decline after 18 months of treatment, indicating its potential in managing conditions like Alzheimer’s disease.
- COVID-19 Protection: A monoclonal antibody concentration of 54-fold the in vitro IC50 is needed to achieve 50% protection from COVID-19. The 95% confidence interval for this concentration ranges from 16 to 183.
- Tixagevimab/Cilgavimab: This combination treatment led to 87% of patients achieving sustained recovery by day 90, compared to 84% in the placebo group, in a trial involving 1,455 patients across 81 sites on four continents. It also showed a nearly 4% lower mortality rate than the placebo group.
Treatment Impact:
- Hospitalization and Emergency Visits: mAb treatment reduced the odds of hospitalization by nearly 50% and emergency department visits by 24%.
Mortality: The odds of 30-day all-cause death were reduced by 86% with mAb treatment. - Treatment Necessity: To prevent hospitalization or death at 30 days, 42 patients needed to be treated with mAb.
- Adverse Events: Only 0.2% of patients experienced any adverse event from mAb treatment.
Usage During the Omicron Era:
- Only 7.6% of patients received sotrovimab, even though 25% were diagnosed in the Omicron era, highlighting potential gaps in treatment application during specific pandemic phases.
Emerging Trends
- Expansion in Treatment Scope: Monoclonal antibodies (mAbs) are increasingly being used beyond their initial applications, treating conditions ranging from cancers to infectious diseases like COVID-19.
- Focus on High-Risk Patients: Studies show a critical need for prioritizing mAbs for high-risk patients to maximize the effectiveness of treatments, especially in managing COVID-19.
- Early Intervention: Early administration of mAbs in the disease process can significantly reduce severity and improve outcomes, which is particularly noted in COVID-19 treatment protocols.
- Combination Therapies: There’s a growing interest in combining monoclonal antibodies with other forms of treatment to enhance efficacy, particularly in the treatment of infectious diseases and in oncology.
- Increased Accessibility and Approval: The FDA has been actively approving mAbs for emergency use, especially for treating mild to moderate COVID-19, to expand access to these crucial therapies.
- Resistance Monitoring: With the continuous evolution of pathogens like the SARS-CoV-2 virus, there is an increased focus on monitoring resistance to mAb treatments and adapting strategies accordingly.
- Innovative Delivery Systems: The development of new delivery systems for mAbs is underway, aiming to improve the efficiency and reduce the complexity of administering these therapies.
- Enhanced Production Techniques: Advances in biotechnology have improved the scalability and reduced the costs of producing monoclonal antibodies, making them more accessible worldwide.
- Tailored Therapies: There is an ongoing trend towards personalizing monoclonal antibody therapies based on individual patient profiles to enhance treatment outcomes and minimize side effects.
- Education and Awareness: Efforts are increasing to educate healthcare providers and patients about the benefits and potential of monoclonal antibodies, ensuring wider acceptance and optimized use.
Use Cases
- COVID-19 Management: mAbs are effectively used to reduce symptoms and prevent severe complications in COVID-19, especially when administered early after diagnosis to high-risk patients.
- Chronic Pain Treatment: Research is exploring mAbs as potential non-addictive treatments for chronic pain, targeting specific nerve channels to block pain transmission without the risks associated with opioids.
- Cancer Therapy: mAbs are a cornerstone in the treatment of various cancers, targeting cancer cells specifically and sparing healthy cells, which minimizes side effects compared to traditional chemotherapy.
- Autoimmune Diseases: For conditions like rheumatoid arthritis and lupus, mAbs can selectively modulate the immune system to reduce inflammation and halt disease progression.
- Preventive Measures: Beyond treatment, mAbs are being investigated for their preventive potential against infections like respiratory syncytial virus (RSV) in children and even for conditions such as Alzheimer’s disease.
- Infectious Diseases: mAbs have been developed to combat other viruses like Ebola, demonstrating their versatility in addressing a range of viral threats.
- Organ Transplantation: They are used to prevent the rejection of transplanted organs by suppressing specific immune responses that could compromise the transplant.
- Ophthalmological Applications: mAbs are used in treating eye diseases that involve immune-mediated mechanisms, such as uveitis.
- Cardiovascular Disease: Certain mAbs target and modulate pathways involved in cholesterol metabolism, offering new approaches to managing heart disease.
- 10. Neurological Conditions: mAbs are being explored to treat multiple sclerosis and migraines, where they help reduce the frequency of episodes and severity of the neurological symptoms.
Conclusion
Monoclonal antibodies (mAbs) have transformed modern medical therapy across various diseases due to their precision in targeting specific antigens. Their expansion from cancer treatments to managing chronic diseases and infectious diseases like COVID-19 highlights their versatility and effectiveness. The ongoing development of mAbs underscores their potential in improving patient outcomes, reducing hospitalizations, and enhancing quality of life. With technological advancements enhancing production and accessibility, the future of monoclonal antibodies looks promising in providing tailored, efficient, and impactful medical treatments across a broad spectrum of health conditions.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



