Overview
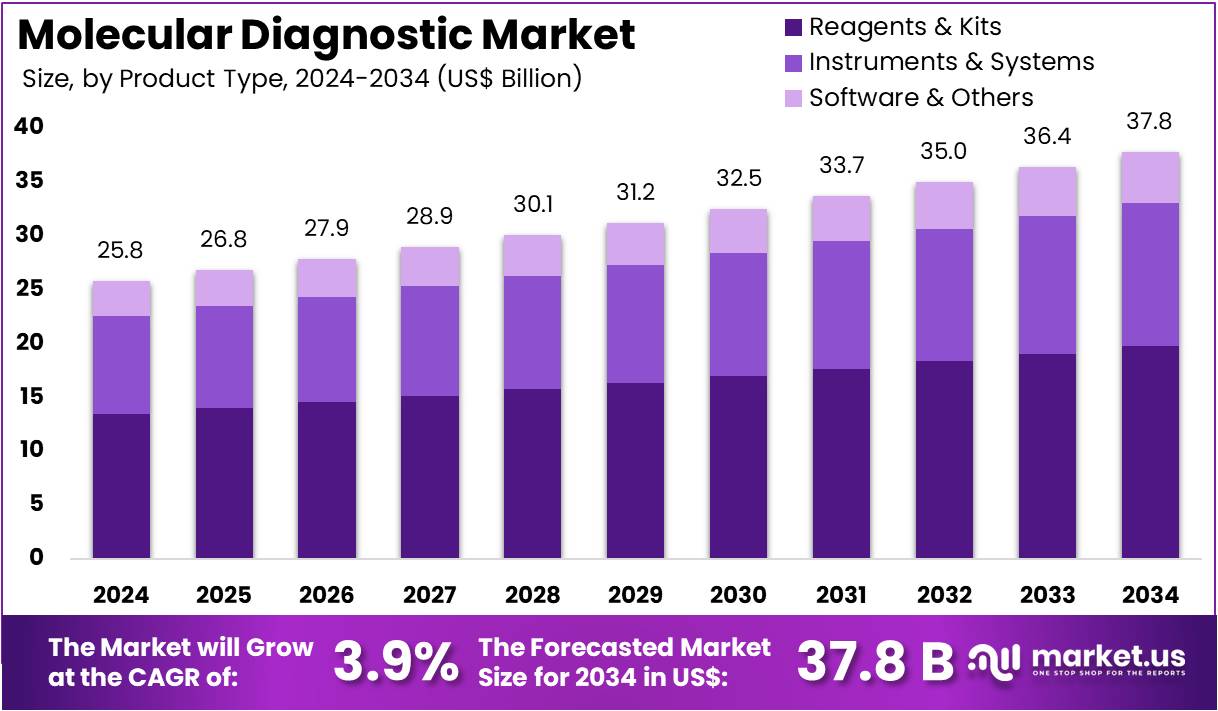
New York, NY – August 26, 2025: The Molecular Diagnostics Market is projected to reach US$ 37.8 billion by 2034, up from US$ 25.8 billion in 2024, growing at a CAGR of 3.9% (2025–2034). North America holds a dominant position with over 41.9% share, valued at US$ 10.8 billion in 2024. Growth is driven by demographic shifts, particularly the rising elderly population, which is highly vulnerable to chronic and infectious diseases. Molecular diagnostics provide early and precise detection, making them essential in managing healthcare needs. Their integration into routine clinical practice is further supported by increasing demand for point-of-care (POC) testing and decentralized healthcare solutions.
The growing popularity of self-testing and rapid diagnostic tools is a major catalyst for expansion. Portable RT-PCR devices and compact diagnostic machines are making testing more accessible, enabling accurate results without specialized training. This reflects the broader trend of moving diagnostics from central laboratories to smaller clinics and even homes. Additionally, the burden of noncommunicable diseases such as cardiovascular conditions, cancers, and diabetes is fueling demand for advanced molecular testing. For instance, the CDC reported 42 million U.S. adults living with diabetes in 2021, highlighting the urgent need for reliable and rapid testing solutions in chronic disease management.
Public health challenges and government initiatives are strengthening adoption. Programs such as Texas’ HPV self-collection pilot (2024) demonstrate the value of POC testing in underserved communities. Similarly, the National Institutes of Health (NIH) continues to fund research in infectious disease surveillance and personalized medicine. These initiatives emphasize molecular diagnostics as vital for early detection and improved treatment strategies. The role of public-private partnerships is becoming increasingly important, enabling faster integration of innovative solutions into healthcare systems while improving accessibility and affordability.
Continuous innovation in molecular diagnostic technologies is reshaping the market. Techniques such as real-time PCR, isothermal amplification, and whole-genome sequencing are now faster, cheaper, and more reliable. These advances allow laboratories to process multiple tests simultaneously with higher accuracy. The importance of rapid molecular diagnostics was underscored during the COVID-19 pandemic and remains high amid rising antimicrobial resistance and seasonal outbreaks. Regulatory agencies are also providing clearer pathways for validation and emergency approvals, accelerating market adoption. Investments in laboratory capacity, genomic surveillance, and workforce training further strengthen the future outlook. Collectively, these efforts are positioning molecular diagnostics as a cornerstone of modern healthcare.
Key Takeaways
- In 2024, the molecular diagnostics market generated US$ 25.8 billion revenue and is forecasted to reach US$ 37.8 billion by 2034.
- The overall market growth is being sustained at a compound annual growth rate (CAGR) of 3.9% over the projected forecast period.
- Within product types, reagents and kits dominated the segment in 2023, holding a commanding market share of 52.4%.
- Instruments and systems, alongside software and other categories, comprised the remaining product type shares within the global molecular diagnostics industry.
- In terms of technology, polymerase chain reaction (PCR) remained the most widely used, capturing a substantial 44.8% share of the market.
- Among applications, infectious disease testing stood as the dominant contributor, generating the highest revenue share of 49.7% in the overall market.
- The test location segment revealed central laboratories as the leader, accounting for 55.9% of the global market’s revenue share.
- Point-of-care testing and self-testing or over-the-counter options followed, but with smaller contributions compared to centralized laboratory testing facilities.
- Geographically, North America emerged as the leading regional market, securing a strong share of 41.9% in 2023.
- The region’s leadership was driven by advanced healthcare infrastructure, higher diagnostic adoption, and robust presence of key molecular diagnostic companies.
Regional Analysis
In 2024, North America retained leadership in the molecular diagnostics market, securing the largest revenue share of 41.9%. This strong position was primarily attributed to the rising adoption of molecular diagnostics, recognized for their high accuracy, sensitivity, and specificity. The demand for advanced diagnostic solutions has continued to increase across the region. The emphasis on early and reliable disease detection has reinforced the role of molecular diagnostics as a preferred choice in both clinical and research settings.
Genetic testing demand significantly contributed to market expansion, particularly in oncology and other complex diseases. Personalized medicine has gained traction, supported by the region’s advanced healthcare infrastructure. The American Cancer Society projected over 2 million new cancer cases in the United States for 2024, underlining the critical disease burden. This high incidence drove the need for molecular diagnostics for early detection, precise staging, and customized therapies, strengthening their integration into clinical practices.
The presence of robust research and development infrastructure further accelerated the adoption of molecular diagnostic technologies in North America. A large concentration of leading industry players actively engaged in innovation contributed to this progress. These companies have continually introduced advanced molecular testing solutions. Their efforts are supported by strategic collaborations with research institutions and healthcare providers. The resulting innovations have enhanced diagnostic precision, widened clinical applications, and provided a strong competitive advantage for the regional market.
Favorable regulatory and reimbursement frameworks further bolstered the regional market’s growth. The US Food and Drug Administration (FDA) has offered clear approval pathways for molecular diagnostic tests, ensuring faster availability of new solutions. A notable example was the FDA approval of Myriad Genetics’ BRACAnalysis CDx test in 2022. Additionally, reimbursement expansions by the Centers for Medicare & Medicaid Services (CMS) improved patient access to molecular tests. This regulatory clarity and reimbursement support fostered innovation and ensured wider clinical adoption across healthcare systems in the region.
Segmentation Analysis
The reagents and kits segment accounted for 52.4% of the molecular diagnostics market and is expected to maintain growth. These products are vital for accurate molecular diagnostic testing, enabling pathogen detection, genetic analysis, and biomarker identification. Their role in infectious disease diagnosis, oncology, and genetic testing ensures sustained demand. The rise of personalized medicine, coupled with improved test accuracy, is expected to drive further adoption. Growing healthcare needs for efficient, reliable, and rapid diagnostics continue to reinforce the importance of reagents and kits in this market.
Polymerase chain reaction (PCR) technology held the largest market share of 44.8% and is expected to remain dominant. PCR is valued for its ability to amplify DNA and RNA sequences with precision, making it indispensable in infectious disease detection, oncology, and genetic testing. The COVID-19 pandemic accelerated its global adoption, with PCR established as the gold standard for virus detection. Continuous advancements, including real-time and digital PCR, are lowering costs and improving speed, ensuring its widespread use across clinical and research applications in the coming years.
Infectious disease diagnostics represented 49.7% of the molecular diagnostic market, reflecting the rising global need for rapid and precise testing. The segment is driven by the increasing prevalence of respiratory, gastrointestinal, and sexually transmitted infections. Technologies such as PCR and sequencing enhance diagnostic accuracy and turnaround times. The COVID-19 pandemic highlighted the value of molecular diagnostics in outbreak management and innovation. Moreover, global health strategies focused on disease surveillance and containment are projected to further accelerate the demand for infectious disease testing solutions.
Central laboratories accounted for 55.9% of the molecular diagnostic market by test location and are expected to retain their lead. These laboratories are equipped with advanced technology and skilled personnel to conduct complex diagnostic tests at scale. Their capacity for high-throughput testing supports oncology, infectious disease, and genetic diagnostics. Growing demand for precision and efficiency in diagnostics is reinforcing their role. The expansion of testing capabilities within central laboratories is anticipated to meet the increasing needs of healthcare providers worldwide, thereby sustaining their dominance in the market.
Conclusion
The molecular diagnostics market is moving toward steady growth as healthcare systems shift to more accurate, early, and accessible testing methods. Rising cases of chronic and infectious diseases, combined with the growing elderly population, are pushing the demand for advanced diagnostic solutions. The adoption of portable and point-of-care tools is making testing faster and more convenient, even outside traditional laboratory settings. Innovation in technologies such as PCR, sequencing, and isothermal amplification continues to expand the scope of clinical applications. Supportive government programs, public-private collaborations, and improved reimbursement policies are further strengthening adoption. As a result, molecular diagnostics are becoming a central pillar in modern healthcare and personalized medicine.
View More
Point-of-Care Molecular Diagnostics Market || Advanced Molecular Nuclear Imaging Market || Molecular Biology Enzymes Market || Molecular Interaction Analyzer Market || Molecular Imaging Market || PCR Molecular Diagnostics Market || Molecular Cytogenetics Market || Core Clinical Molecular Diagnostics Market


