Table of Contents
Introduction
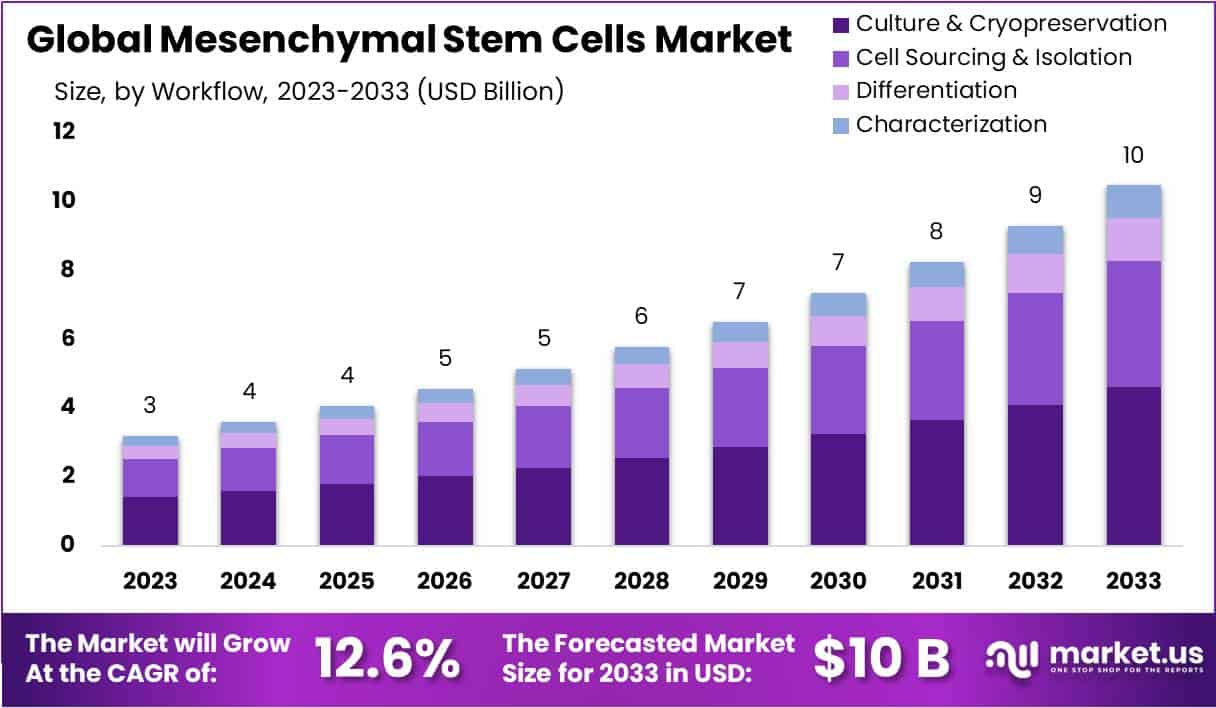
The Mesenchymal Stem Cells (MSC) Market is projected to grow significantly, reaching approximately USD 10 billion by 2033, up from USD 3 billion in 2023. This expansion is expected to occur at a robust Compound Annual Growth Rate (CAGR) of 12.6% from 2024 to 2033. The increasing adoption of MSCs in regenerative medicine and their therapeutic potential across various medical applications are key drivers of this growth. Additionally, continuous advancements in research and regulatory approvals have strengthened the market’s development, positioning MSCs as essential components in innovative medical treatments.
The therapeutic versatility of MSCs has fueled market demand. These adult stem cells can differentiate into multiple cell types, including bone, cartilage, and fat cells, making them highly valuable for regenerative therapies. Their ability to modulate immune responses has further expanded their applications in treating autoimmune diseases, cardiovascular disorders, and orthopedic conditions. Furthermore, MSCs are being explored for their role in cancer therapy, where they can naturally target tumor cells and deliver therapeutic agents, offering a novel and promising approach to oncology treatment.
Recent research has identified key regulatory factors influencing MSC growth and differentiation. Notably, the tumor suppressor protein p53 plays a crucial role in determining their efficacy and therapeutic potential. Understanding these regulatory mechanisms has enhanced the optimization of MSC-based therapies, leading to improved clinical outcomes. Additionally, advancements in cell expansion techniques and bioprocessing methods have increased the scalability of MSC production, making them more accessible for widespread clinical applications.
Regulatory approvals have significantly contributed to market expansion. A key milestone was the FDA approval of Mesoblast’s Ryoncil for treating children with steroid-refractory acute graft-versus-host disease. This approval highlights the growing acceptance of MSC-based therapies and encourages further investment in clinical research. Regulatory agencies across North America, Europe, and Asia-Pacific have also streamlined approval processes, facilitating the commercialization of MSC therapies in various medical fields. The increasing number of clinical trials and favorable regulatory policies are expected to drive further market growth.
The mesenchymal stem cell market is poised for significant expansion, driven by their therapeutic versatility, advancements in research, and regulatory approvals. The increasing adoption of MSCs in regenerative medicine and their potential applications in cancer therapy further enhance market prospects. With ongoing developments in cell processing technologies and favorable regulatory frameworks, the MSC market is expected to witness sustained growth, reinforcing its role in modern medical treatments.
Key Takeaways
- Market Expansion: The MSC market is projected to reach USD 10 billion by 2033, growing at a strong 12.6% CAGR, driven by research and therapy adoption.
- Market Dynamics: Increased MSC research and technological advancements propel investments, fostering market expansion and encouraging wider adoption of stem cell-based therapies.
- Therapeutic Significance: MSCs play a vital role in regenerative medicine, addressing orthopedic, cardiovascular, autoimmune disorders, wound healing, and potential applications in diabetes and neurology.
- Leading Workflow Segment: Culture & Cryopreservation dominates with a 44.1% share, ensuring MSC viability, preservation, and extended functionality throughout their research and clinical use.
- Primary Isolation Sources: Bone Marrow leads with a 25.1% market share due to its rich MSC content, accessibility, and established use, followed by Cord Blood and Peripheral Blood.
- Dominant Indication: Cardiovascular Diseases represent the leading indication with a 23.2% market share, emphasizing MSCs’ significance in treating heart-related conditions and improving patient outcomes.
- Application Priority: Disease Modeling holds a 23.2% share, allowing researchers to study various medical conditions in controlled laboratory settings, enhancing therapeutic discoveries.
- Key Market Drivers: Regulatory approvals and clinical trial advancements boost market confidence, accelerating the adoption and commercialization of MSC-based treatments.
- Market Restraints: High costs and limited reimbursement options create financial barriers, restricting patient accessibility and slowing widespread MSC therapy adoption.
- Regional Market Leader: North America dominates with a 45.2% share (USD 1.44 billion), driven by advanced research facilities, strong healthcare infrastructure, and proactive medical technology adoption.
- Emerging Trends: Innovations in exosome therapies, AI integration, and regenerative medicine advancements continue shaping the market, with North America maintaining regional leadership.
Emerging Trends
Mesenchymal Stem Cells (MSCs) are becoming a major focus in medical research. Their ability to repair tissues and modulate the immune system makes them valuable for various treatments. Scientists are actively exploring new ways to use MSCs in regenerative medicine and disease management.
- Advancements in Clinical Applications: MSCs show promise in treating several health conditions. Researchers are studying their effects on autoimmune diseases like multiple sclerosis and systemic lupus erythematosus. These conditions cause the immune system to attack healthy tissues. MSCs may help by reducing inflammation and repairing damage. In cardiovascular diseases, MSCs offer potential solutions. Studies suggest they can regenerate heart tissue and improve heart function. This could be a breakthrough for patients with heart failure. As research continues, more clinical trials are expected to validate these benefits.
- Regulatory Approvals and Market Growth: The regulatory landscape for MSC-based therapies is evolving. Governments and health agencies are approving more treatments based on MSCs. In December 2024, the U.S. FDA approved Ryoncil, an MSC-based therapy. It is used to treat graft-versus-host disease in children, a serious complication of stem cell transplants. With such approvals, the MSC market is expanding. Companies are investing in research and commercialization. The growing demand for regenerative medicine is driving further advancements. As regulations become clearer, more therapies are likely to reach the market.
- Ethical and Economic Considerations: Despite the potential benefits, MSC therapies raise ethical and financial concerns. Stem cell treatments can be costly, with some procedures reaching $25,000. In contrast, donors who provide stem cells receive only about $200. This pricing disparity sparks ethical debates about fair compensation and accessibility. Additionally, there are concerns about treatment affordability. Many patients cannot access MSC therapies due to high costs. This has led to discussions on insurance coverage and government support. Ensuring ethical and affordable access to MSC treatments will be essential for long-term adoption.
Use Cases
Mesenchymal stem cells (MSCs) are being used in various medical treatments. Their ability to repair tissues and regulate immune responses makes them valuable in modern medicine. Below are some key applications of MSCs.
- Treatment of Graft-Versus-Host Disease (GVHD): GVHD is a serious complication that can occur after a bone marrow transplant. It happens when the donor’s immune cells attack the recipient’s body. Some patients do not respond to standard steroid treatments. For them, MSC-based therapies offer hope. The drug Ryoncil is an approved MSC therapy. It is used to treat children with steroid-resistant GVHD. Clinical studies show that MSCs help regulate the immune system. They also reduce inflammation and tissue damage. This treatment is improving survival rates and quality of life for affected patients.
- Cardiac Tissue Regeneration: Heart disease and heart failure are leading causes of death worldwide. When heart muscle gets damaged, it does not regenerate easily. MSCs offer a new solution. Scientists have developed stem cell patches containing MSCs. These patches are applied to damaged heart tissue. Over time, they help rebuild heart muscle. Patients with heart failure show better heart function after this treatment. Research suggests that MSCs improve blood flow and reduce scar formation. This approach could become a mainstream treatment for heart disease in the future.
- Treatment of Sports Injuries: Athletes often suffer from serious injuries. Some injuries do not heal properly, affecting performance and careers. MSC therapy is emerging as a promising option. Many athletes seek these treatments abroad, where they are more widely available. For example, a former Celtic FC goalkeeper traveled to Colombia for MSC therapy. He had a recurring thigh injury that did not heal with conventional treatments. After receiving MSC injections, he successfully recovered. His case highlights the potential of MSCs in sports medicine. Research suggests that MSCs accelerate healing and reduce inflammation in damaged tissues.
- Treatment of Autoimmune Diseases: Autoimmune diseases occur when the immune system attacks the body’s own cells. Conditions like multiple sclerosis (MS) can cause severe disability. Traditional treatments focus on managing symptoms, but MSCs could offer a long-term solution. Studies show that MSCs can modulate immune responses and reduce inflammation. They also help repair damaged tissues. In early trials, MS patients treated with MSCs experienced fewer relapses and improved neurological function. While more research is needed, these findings suggest that MSC therapy could become a breakthrough treatment for autoimmune diseases.
Conclusion
The mesenchymal stem cell market is growing rapidly due to advancements in regenerative medicine and increasing clinical applications. These stem cells offer promising treatments for various conditions, including heart disease, autoimmune disorders, and sports injuries. Regulatory approvals and ongoing research are driving market expansion, making MSC-based therapies more accessible. However, challenges like high costs and limited reimbursement options remain barriers to widespread adoption. Despite these challenges, continuous innovation in cell processing and therapeutic applications is expected to support market growth. As demand for regenerative treatments rises, the MSC market will play a key role in shaping the future of modern medicine, offering new hope for patients worldwide.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



