Table of Contents
Introduction
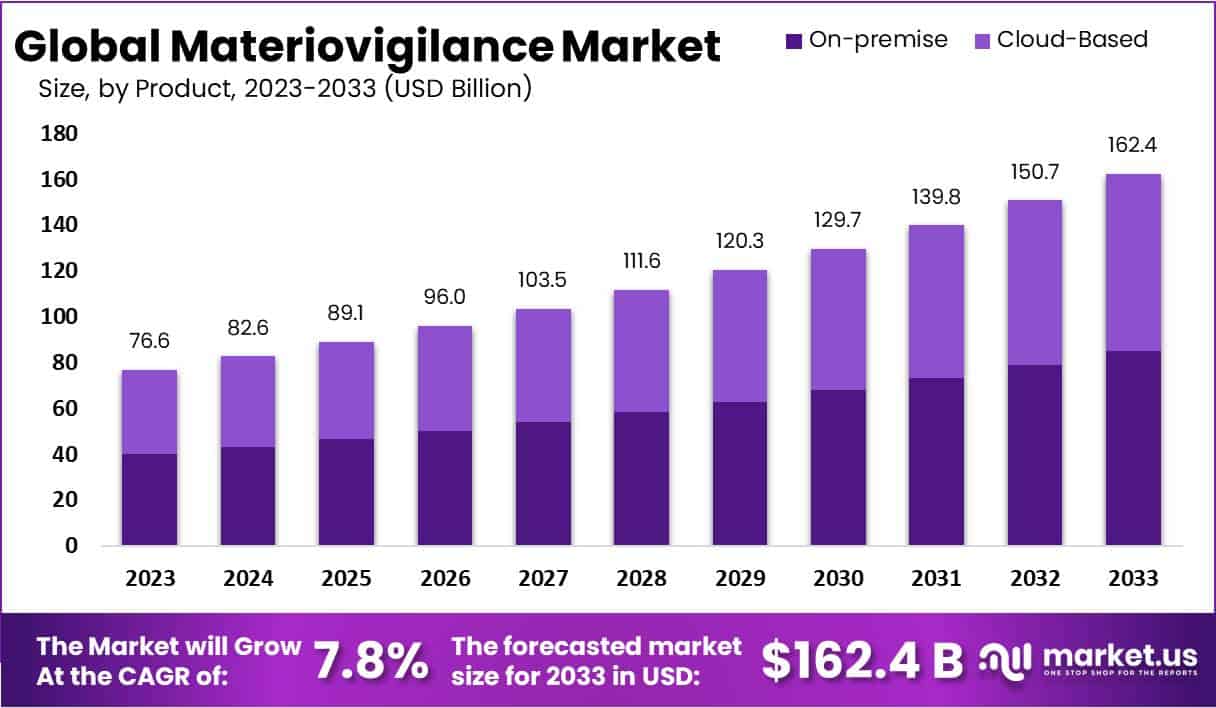
The global Materiovigilance Market is poised for substantial growth, projected to reach approximately US$ 162.4 billion by 2033, up from US$ 76.6 billion in 2023, with a steady CAGR of 7.8% during the forecast period of 2024 to 2033. This expansion is largely fueled by heightened global emphasis on patient safety and stringent quality assurances for medical devices.
Regulatory initiatives play a crucial role in this sector’s growth. For example, the Materiovigilance Programme of India (MvPI) exemplifies how regulatory frameworks are being enforced to ensure that medical devices’ benefits significantly outweigh the risks. Such programs enhance patient safety by fostering systematic adverse event monitoring and reporting, emphasizing the importance of regulatory compliance in driving market growth.
Technological advancements are crucial in enhancing the tracking and analysis of medical device data. The adoption of advanced technologies in healthcare facilitates improved device safety, supports early detection of safety issues, and enhances communication between users and regulatory bodies. This technological integration is vital for ensuring rapid responses to safety concerns, thus bolstering the market’s expansion.
Additionally, there is a growing focus on educating healthcare providers about the importance of adverse event reporting. Through continuous medical education (CME) and various training programs, healthcare professionals are becoming more adept at using materiovigilance systems effectively. Increased awareness and training lead to better compliance and more robust data collection, further contributing to the sector’s growth.
The market also benefits from international collaboration and public health prioritization. Global cooperation in materiovigilance helps harmonize safety standards across countries, essential for managing international device safety and compliance. Moreover, with public health policies increasingly emphasizing the safe use of medical devices, the materiovigilance sector becomes pivotal in achieving broader health objectives, thus driving market growth.
In the corporate realm, strategic acquisitions such as Honeywell International Inc.’s purchase of Sparta Systems in December 2020 for $1.3 billion highlight the sector’s dynamic nature. This acquisition integrates Sparta’s quality management software into Honeywell’s offerings, enhancing life sciences compliance and market readiness. Additionally, Xybion Corporation’s acquisition of Autoscribe Informatics in November 2023 expands its digital capabilities in life sciences and related industries, marking significant growth in global markets. These developments underscore the continuous evolution and importance of materiovigilance in ensuring medical device safety and efficacy.
Key Takeaways
- The Materiovigilance Market is projected to grow at a 7.8% CAGR, reaching USD 162.4 billion by 2033 from USD 76.6 billion in 2023.
- In 2023, On-premise delivery mode led with a 52.3% market share, valued for its reliability and localized control capabilities.
- Cloud-Based delivery closely followed, appreciated for its flexibility and scalability in the market.
- Diagnostic Applications topped the application segment with a 32.6% market share in 2023, critical for ensuring diagnostic material safety.
- Other significant applications included Therapeutic, Surgical, and Research, each contributing notably to the market.
- Original Equipment Manufacturers (OEM) dominated the end-user segment, holding a 46.8% market share in 2023.
- Contract Research Organizations (CROs) and Business Process Outsourcing (BPO) sectors also played vital roles in the market.
- Key growth drivers include stringent regulatory standards and increasing adverse event incidences.
- North America was the leading region in 2023, with a 46.2% market share and a value of USD 35.3 billion.
- Potential growth opportunities exist in the rising adoption of cloud solutions, emerging markets, and the integration of blockchain technology.
Materiovigilance Statistics
- 2023 Market Size: US$ 76.6 billion, split between on-premise and cloud-based solutions.
- 2024 Market Size: US$ 82.6 billion, indicating growth in technology adoption.
- 2025 Market Size: US$ 89.1 billion, showing a steady market expansion.
- 2026 Market Size: US$ 96.0 billion, as the market continues to evolve.
- 2027 Market Size: US$ 103.5 billion, reflecting increasing global demand.
- 2028 Market Size: US$ 111.6 billion, with both segments expanding.
- 2029 Market Size: US$ 120.3 billion, driven by advancements in cloud technology.
- 2030 Market Size: US$ 129.7 billion, showcasing sustained growth.
- 2031 Market Size: US$ 139.8 billion, with significant contributions from cloud solutions.
- 2032 Market Size: US$ 150.7 billion, as market penetration deepens.
- 2033 Market Size: US$ 162.4 billion, forecasted to achieve significant milestones.
- Annual Growth Rate: The market will grow at a Compound Annual Growth Rate (CAGR) of 7.8%.
Emerging Trends
- Enhanced Adverse Event Reporting Systems: The enhancement of adverse event reporting systems represents a notable trend in materiovigilance. With the integration of advanced technologies, these systems now incorporate mobile apps and online platforms that streamline the process of reporting issues associated with medical devices. This technological advancement facilitates more efficient and comprehensive data collection, contributing significantly to the improvement of patient safety and device efficacy.
- Increased Government Involvement: There is a noticeable increase in governmental participation in materiovigilance. Authorities are setting up specialized programs dedicated to monitoring the safety and effectiveness of medical devices. The primary goal of these programs is to protect public health by ensuring that medical devices fulfill their intended benefits while minimizing potential risks.
- Global Collaboration for Safety Standards: A key emerging trend in materiovigilance is the push towards international cooperation. Countries around the world are joining forces to harmonize safety standards and exchange information on medical device safety. This global collaboration aids in establishing more stringent and effective safety protocols worldwide.
- Focus on Training and Awareness: The importance of training and raising awareness about the significance of reporting adverse events is increasingly recognized. Educational initiatives targeting healthcare providers and the general public are intensifying. This trend aims to improve the quality and quantity of the reports submitted, which is essential for enhancing patient safety outcomes.
- Development of Specialized Materiovigilance Centers: The creation of specialized materiovigilance centers marks a growing trend. These centers are dedicated to analyzing data from adverse events and developing safety measures. Their role is crucial in boosting the effectiveness of materiovigilance systems, ultimately leading to safer medical device usage and better patient outcomes.
Use Cases
- Real-Time Data Monitoring: In materiovigilance, the application of real-time data monitoring tools is transformative. These tools enable the immediate identification and management of adverse events linked to medical devices. By deploying real-time monitoring, healthcare professionals can swiftly respond to potential issues, substantially reducing the risks to patients. This immediate response capability is crucial in preventing minor device malfunctions from escalating into serious health complications, thereby enhancing patient safety and trust in medical technologies.
- Risk Management and Mitigation: Materiovigilance systems play a pivotal role in the healthcare industry by systematically identifying patterns and trends in device malfunctions and adverse reactions. This data is crucial for developing effective risk management strategies. With this proactive approach, potential risks can be mitigated before they evolve into significant health concerns. This strategic risk management not only protects patients but also reinforces the reliability of medical devices in clinical settings.
- Policy Development: The insights gathered from materiovigilance activities are invaluable for regulatory bodies tasked with overseeing medical device safety. These data points are instrumental in informing and revising policies that govern the use of medical technologies. As medical devices become more advanced, having a dynamic and responsive regulatory framework is essential. This ensures that guidelines and policies evolve in tandem with technological advancements, maintaining high safety standards.
- Educational Programs: Materiovigilance systems provide a wealth of data that is essential for educating healthcare providers about the risks associated with medical devices. By leveraging this information, educational programs can be developed to enhance the awareness and vigilance of healthcare professionals regarding device-related risks. Educating providers on the importance of reporting adverse events helps in cultivating a culture of safety and proactive risk management within healthcare facilities.
- Quality Improvement: The continuous feedback loop provided by materiovigilance systems is a critical component in the iterative process of medical device manufacturing. This feedback allows manufacturers to refine and enhance the design and quality of their products. By integrating these insights, manufacturers can ensure that their medical devices are not only compliant with regulatory standards but also meet the highest safety and effectiveness criteria, ultimately benefiting patient care.
Key Players Analysis
AssurX
AssurX is recognized as a prominent player in the materiovigilance sector, focusing on enhancing compliance and efficiency in medical and pharmaceutical environments. Their electronic Quality Management Systems (eQMS) are particularly valued for their ease of use and configurability, which enables tailored solutions for complex regulatory requirements in healthcare. Companies like MIMEDX have leveraged AssurX to transition from manual to automated systems, significantly improving the management of quality events and compliance reporting. This shift not only streamlines operations but also aids in maintaining stringent safety standards for medical products, which is critical in the materiovigilance landscape.
Sparta Systems
Sparta Systems, a notable player in the Materiovigilance sector, enhances the safety and effectiveness of medical devices through its advanced quality management software. This software, part of a broader SaaS platform, significantly contributes to the digitalization and standardization of quality processes for medical devices. Since being acquired by Honeywell, Sparta has integrated its capabilities with Honeywell Forge and Experion Process Knowledge System. This integration aims to advance global growth and improve quality assurance, regulatory compliance, and manufacturing processes in the life sciences sector. The company’s innovative approach helps detect manufacturing anomalies and ensures the quality and safety of medical devices, crucial for patient safety.
Oracle Corporation
Oracle Corporation is making significant contributions to the Materiovigilance sector by leveraging its technological expertise to offer advanced solutions. These solutions focus on enhancing the efficiency of materiovigilance systems, which are crucial for the monitoring and reporting of adverse events related to medical devices. Oracle’s integrated systems play a key role in addressing the evolving needs of this market by providing real-time data sharing, scalability, and accessibility. This not only supports the industry’s demand for effective adverse event reporting but also aligns with the trend towards more patient-centric materiovigilance practices. The company’s involvement is pivotal in fostering the integration of technologies such as AI and blockchain, which are vital for ensuring data integrity and security within the materiovigilance framework.
Xybion Corporation
Xybion Corporation is a prominent player in the materiovigilance market, offering tailored solutions that align with regulatory requirements. Their digital cloud platform specializes in integrating quality, compliance, and data integrity, specifically designed to meet the needs of the medical device manufacturing industry. Xybion emphasizes reducing compliance risks and operational costs, which are critical in the heavily regulated medical sector. Their approach not only supports regulatory adherence but also enhances efficiency and cost-effectiveness, achieving up to a 30% reduction in total ownership costs. This makes them a key contributor to advancing materiovigilance practices, particularly in environments that demand high levels of data security and regulatory compliance.
Sarjen Systems
Sarjen Systems Pvt. Ltd. plays a significant role in the materiovigilance sector through its advanced software solutions designed for medical device vigilance. Their platform, PvEdge®, is a comprehensive pharmacovigilance safety database that manages safety data across various pharmaceutical products, including medical devices. It helps device manufacturers with post-marketing surveillance to identify and report problems or risks associated with medical devices, thereby ensuring compliance with global safety regulations like those from the US FDA and EU guidelines.
The software streamlines the collection and analysis of adverse events, offering tools for incident reporting, document management, and regulatory compliance, making it a valuable tool for organizations aiming to maintain high standards of safety and quality in medical devices. Sarjen’s system is recognized for its robustness in handling sensitive data while ensuring data privacy and regulatory adherence, crucial for effective materiovigilance practices.
MDI Consultants
MDI Consultants is a renowned leader in the healthcare industry, specializing in regulatory compliance services tailored for the medical device, pharmaceutical, and food sectors. With expertise grounded in U.S., European, and Canadian regulations, MDI supports companies in achieving compliance with rigorous standards set by bodies like the FDA. Their services are vital for navigating the complex regulatory frameworks that companies must adhere to, ensuring that medical devices, drugs, and food products meet safety and quality standards before reaching the market.
MDI’s team is notable for its depth of experience, including former FDA officials and industry experts who offer insights into FDA compliance, clinical trial management, and quality system compliance. This rich expertise enables MDI to assist companies not only in meeting regulatory requirements but also in maintaining ongoing compliance, which is critical for the lifecycle of healthcare products. MDI’s comprehensive services include everything from initial market entry strategies such as FDA 510(k) submissions to ongoing regulatory support and audit defense, positioning them as a crucial ally for healthcare companies aiming to succeed in highly regulated markets.
QVigilance
QVigilance is a prominent provider in the materiovigilance sector, focusing on medical device vigilance which includes the collection, assessment, reporting, and trend identification related to incidents involving medical devices. Their services are crucial for ensuring patient safety by identifying potential risks and preventing their recurrence. QVigilance offers comprehensive support for both pre- and post-market phases of medical devices, ensuring compliance with regulatory requirements such as those set by the FDA and EMA. Their materiovigilance services include safety data management, adverse device effect processing, and regulatory compliance reporting, which are essential for medical device manufacturers to maintain high safety standards.
Qserve
Qserve plays a pivotal role in the materiovigilance sector, focusing on regulatory compliance and quality assurance for medical devices and in-vitro diagnostics. As a consultancy firm, Qserve is dedicated to ensuring that medical device manufacturers meet stringent regulatory standards, which are crucial for patient safety and product reliability. They assist companies in navigating the complex landscape of medical device regulations, such as the European Union Medical Device Regulation (EU MDR) and In Vitro Diagnostic Regulation (IVDR), by offering services like regulatory strategy development, risk management, and post-market surveillance support.
Qserve’s expertise is especially valuable in addressing challenges posed by increased documentation requirements, stringent clinical evaluations, and the need for continuous post-market surveillance under new regulatory frameworks. Their interim support services provide medical device companies with access to experienced regulatory professionals who help manage the compliance workload, ensuring timely adherence to regulations without overburdening the company’s resources.
ZEINCRO
ZEINCRO is a notable player in the materiovigilance sector, primarily engaging as a Contract Research Organization (CRO) that focuses on clinical trial management, pharmacovigilance, and medical safety. Established in 1998 and headquartered in the UK, ZEINCRO operates across over 20 countries in Central-Eastern and Southern Europe. The company has extensive experience in conducting clinical trials, having successfully completed more than 700 studies. ZEINCRO specializes in a broad range of therapeutic areas and emphasizes patient recruitment and compliance to enhance trial outcomes. This expertise contributes significantly to the materiovigilance market, which involves the safety monitoring of medical devices. The company’s robust approach in these regions sets it apart as a leader in implementing effective clinical trials that meet high safety standards.
Conclusion
In conclusion, the Materiovigilance market is set to experience significant growth, driven by increasing global focus on patient safety and stringent regulatory standards for medical devices. Enhanced technological integration plays a critical role in advancing the tracking and analysis of medical device data, contributing to improved safety outcomes. With governments and regulatory bodies intensifying their oversight through specialized programs and initiatives, and with the growing emphasis on training healthcare providers in adverse event reporting, the market is poised for robust expansion. Furthermore, strategic corporate actions and global collaborations are reinforcing the market’s strength, ensuring continuous improvements in medical device safety and efficacy. This dynamic market environment underscores the essential role of materiovigilance in fostering a safer healthcare landscape.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



