Table of Contents
Overview
New York, NY – Sep 24, 2025 – The Global Malaria Rapid Diagnostic Tests (RDTS) Market size is expected to be worth around USD 726.9 Million by 2033 from USD 464.5 Million in 2024, growing at a CAGR of 5.1% during the forecast period from 2025 to 2033.
The global fight against malaria has been strengthened by the increasing adoption of Malaria Rapid Diagnostic Tests (RDTs). These tests are designed to provide accurate and timely results at the point of care, even in remote and resource-limited settings. The formation of RDTs typically relies on detecting specific antigens derived from malaria parasites in a patient’s blood sample. This process is supported by immunochromatographic techniques that use monoclonal antibodies on a test strip to deliver results within 15–20 minutes.
The adoption of RDTs has been driven by their simplicity, cost-effectiveness, and ability to reduce dependence on microscopy, which requires trained personnel and laboratory infrastructure. The tests have become a cornerstone of malaria control programs, ensuring early diagnosis and treatment while preventing unnecessary use of antimalarial drugs.
Global health organizations and governments continue to emphasize RDT deployment as part of integrated strategies to reduce malaria prevalence. The tests are widely available in rural healthcare centers and have proven crucial in areas where access to laboratory diagnostics remains limited.
Ongoing innovations in sensitivity and specificity are expected to further enhance the reliability of RDTs. With malaria still posing a major health burden, particularly in Africa and Asia, the expansion of rapid diagnostics stands as a vital intervention to support early case detection and to contribute to the World Health Organization’s targets for malaria elimination.
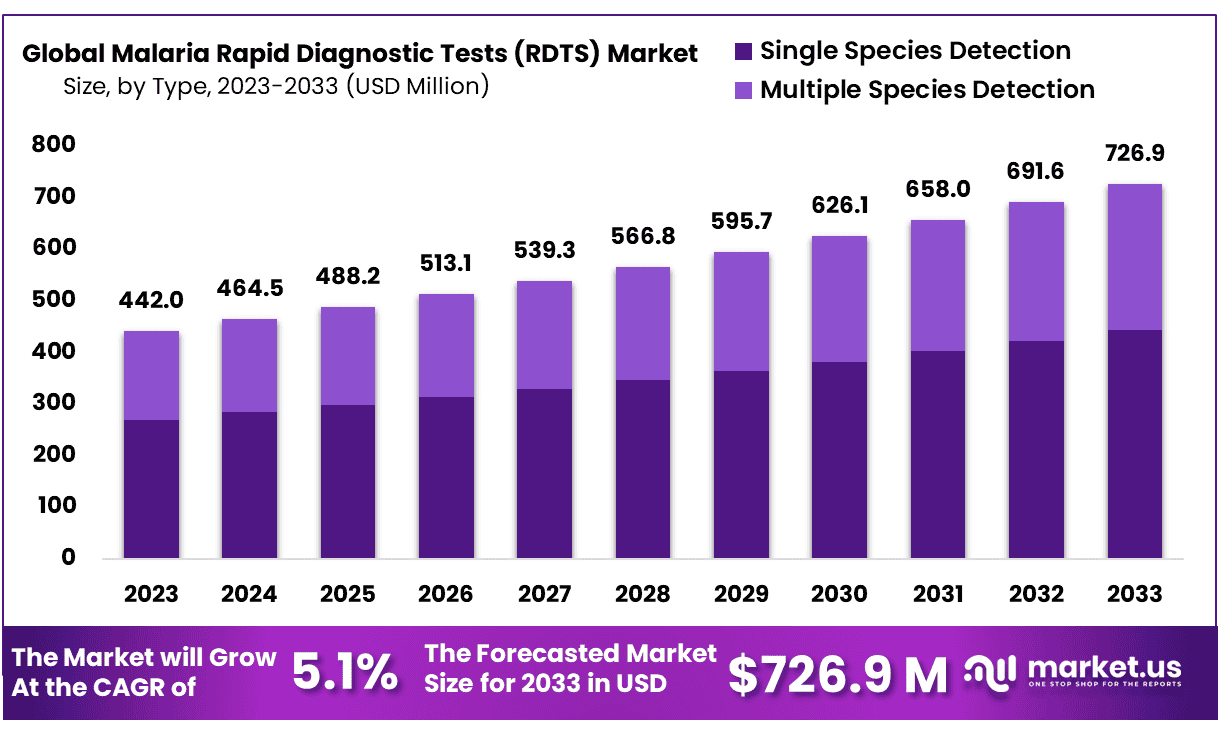
Key Takeaways
- Market Size: Global Malaria Rapid Diagnostic Tests (RDTS) Market size is expected to be worth around USD 726.9 Million by 2033 from USD 464.5 Million in 2024, growing at a CAGR of 5.1% during the forecast period from 2025 to 2033.
- Market Growth: The industry is anticipated to expand at a CAGR of 5.1% during the forecast period of 2024–2033.
- Type Analysis: Single species detection tests dominate the market, accounting for 61.1% of the share.
- End-Use Analysis: Hospitals remain the leading end-users, holding a 54% share of the global market.
- Regional Analysis: The Asia-Pacific (APAC) region is expected to represent 36.4% of the market, with a valuation of USD 160.2 million in 2023.
- Technological Advancements: Continuous innovations are enhancing the sensitivity and specificity of RDTs, strengthening disease management and control measures.
- Government Initiatives: Supportive policies and funding for malaria eradication programs are accelerating the adoption of rapid diagnostic solutions.
- Accessibility: Ongoing initiatives to improve affordability and availability in low-resource regions are driving market penetration.
- Healthcare Integration: The integration of RDTs into primary healthcare systems is enabling faster diagnosis and effective malaria treatment.
- Public Health Impact: Widespread use of RDTs is contributing to a decline in malaria morbidity and mortality, particularly in high-burden countries.
- Private Sector Role: Growing participation of private companies in developing and distributing advanced diagnostic tests is fueling overall market expansion.
Regional Analysis
The Malaria Rapid Diagnostic Tests (RDTs) market is segmented into five key regions, namely North America, Western Europe, Eastern Europe, Asia-Pacific (APAC), and Latin America. Among these, the Asia-Pacific region is projected to account for the largest share, representing 36.4% of the global market and valued at approximately USD 160.2 million in 2023.
The dominance of the APAC region can be attributed to the high prevalence of malaria, driven by favorable climatic conditions for mosquito breeding and the widespread presence of Anopheles gambiae, a primary malaria vector that remains challenging to control. Increasing government initiatives, coupled with international funding for malaria control programs, are further supporting the adoption of RDTs in this region.
Moreover, the rising integration of RDTs into healthcare delivery systems, particularly in rural and resource-limited areas, is enhancing timely diagnosis and treatment. These factors collectively reinforce APAC’s significant role in the malaria diagnostics industry.
Frequently Asked Questions on Malaria Rapid Diagnostic Tests (RDTS)
- What are malaria rapid diagnostic tests (RDTs)?
Malaria rapid diagnostic tests (RDTs) are simple point-of-care tools used to detect malaria infection by identifying parasite-specific antigens in a patient’s blood. They provide results within 15–20 minutes, enabling quick treatment decisions without advanced laboratory facilities. - How do malaria RDTs work?
RDTs work by using immunochromatographic methods that detect malaria parasite antigens, such as HRP2 or pLDH, in a small blood sample. The appearance of a visible colored line indicates a positive result for malaria infection. - Why are malaria RDTs important?
Malaria RDTs are important because they ensure rapid detection, particularly in remote or resource-limited settings where microscopy is unavailable. They reduce misdiagnosis, promote rational drug use, and support global malaria control and elimination programs. - What are the limitations of malaria RDTs?
RDTs may produce false negatives due to low parasite densities or gene deletions like HRP2 deletions. They also cannot quantify parasite load, and performance may vary depending on storage conditions, product quality, and regional parasite strains. - Who commonly uses malaria RDTs?
Malaria RDTs are widely used by healthcare workers, community health volunteers, and international health organizations in endemic regions. They are particularly valuable in rural areas, where access to laboratory diagnostics and skilled personnel is limited. - Which regions dominate the malaria RDTs market?
Sub-Saharan Africa dominates the malaria RDTs market due to the high disease burden and extensive deployment of RDTs in public health programs. Asia-Pacific also contributes significantly, supported by government-led malaria elimination initiatives and large population exposure. - Who are the key players in the malaria RDTs market?
Key market players include Abbott Laboratories, SD Biosensor, Access Bio, Premier Medical Corporation, and Biosynex. These companies focus on product innovation, cost-effectiveness, and distribution partnerships to strengthen their presence in malaria-endemic regions.
Conclusion
The global malaria rapid diagnostic tests (RDTs) market is positioned for steady growth, supported by rising disease prevalence, government initiatives, and international funding. The simplicity, affordability, and efficiency of RDTs make them indispensable in resource-limited settings, where access to laboratory infrastructure is restricted.
Asia-Pacific is emerging as the dominant region due to high malaria incidence and supportive healthcare strategies, while continuous technological advancements are enhancing test accuracy and reliability. With increasing integration into primary healthcare systems and strong contributions from private players, RDTs will remain a cornerstone of malaria control and elimination efforts, reducing morbidity and mortality worldwide.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



