Table of Contents
Overview
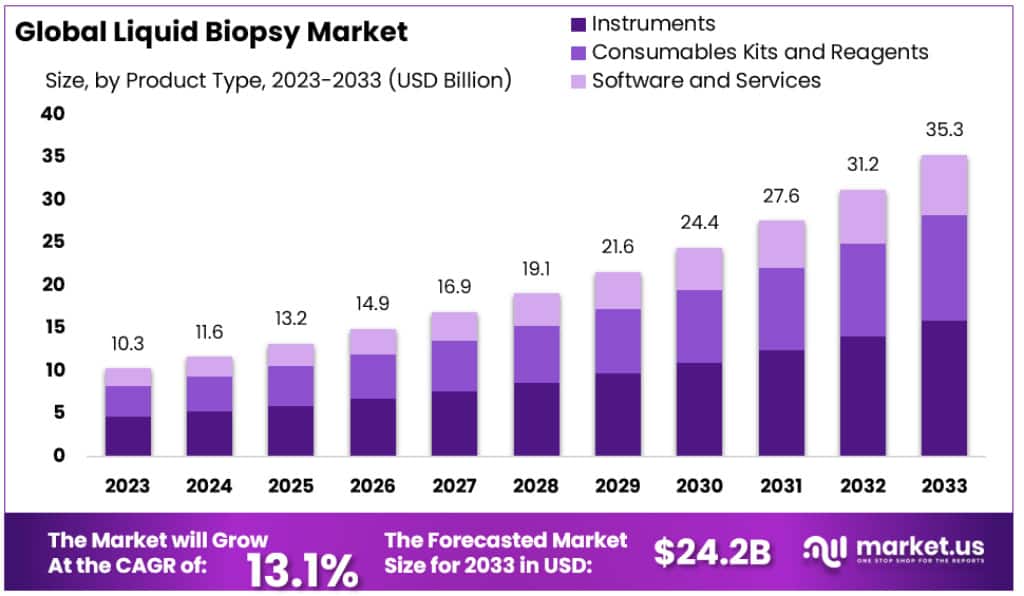
The Global Liquid Biopsy Market is projected to grow from USD 10.3 billion in 2023 to nearly USD 35.3 billion by 2033. This represents a robust CAGR of 13.1% during the forecast period. The expansion is strongly linked to the rising global burden of cancer. According to the World Health Organization, cancer cases are expected to exceed 29 million by 2040. This rising prevalence creates demand for advanced, non-invasive diagnostics, with liquid biopsy emerging as a crucial tool for early detection and disease monitoring.
The increasing preference for non-invasive diagnostic techniques has accelerated the adoption of liquid biopsy. Traditional tissue biopsies are invasive, costly, and involve surgical risks, making them less suitable for repeat testing. In contrast, liquid biopsy provides a safer and more cost-effective solution. Its ability to analyze circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), and exosomes in real time offers clinicians valuable insights into tumor heterogeneity, supporting better disease management and timely intervention.
Technological advancements in genomics and sequencing have also been pivotal. The introduction of next-generation sequencing (NGS), digital PCR, and sophisticated bioinformatics has substantially improved test sensitivity and specificity. These innovations enable the detection of rare genetic mutations and minimal residual disease (MRD), which was previously difficult to track. The advancement of molecular diagnostics has extended the scope of liquid biopsy beyond cancer care, making it relevant in prenatal testing, organ transplant monitoring, and even infectious disease diagnostics.
The broadening of applications outside oncology has further strengthened the market. Non-invasive prenatal testing (NIPT) is gaining popularity as a safe alternative to invasive procedures. In transplant medicine, liquid biopsy supports early identification of organ rejection risks. Additionally, in infectious disease management, liquid biopsy facilitates viral load monitoring. This diversification is expected to reduce the market’s dependency on oncology alone, opening new avenues for growth across multiple healthcare segments worldwide.
Precision Medicine, Collaborations, and Market Enablers
The adoption of precision medicine and personalized therapies has been another strong driver. Liquid biopsy plays an essential role in precision oncology, as it allows clinicians to tailor treatments according to tumor-specific genetic profiles. This aligns with the growing emphasis on targeted therapies and companion diagnostics. Pharmaceutical companies are also integrating liquid biopsy into drug development and clinical trials, particularly for patient stratification and therapy optimization, creating synergies between diagnostics and therapeutics in the healthcare ecosystem.
Rising investments and collaborations are accelerating the commercialization of liquid biopsy technologies. Venture capital firms, biotechnology companies, and government research agencies are channeling substantial funds into the sector. Partnerships between diagnostic companies, pharmaceutical firms, and research institutions are fostering technological innovations and supporting global expansion. These collaborations have led to breakthroughs in assay sensitivity and accessibility, allowing liquid biopsy to transition from a niche diagnostic tool to a mainstream clinical practice in many regions.
Regulatory approvals and supportive reimbursement frameworks are also driving adoption. Regulatory agencies such as the U.S. FDA and the European CE mark authorities are increasingly approving liquid biopsy-based assays. At the same time, reimbursement policies in the United States, Europe, and Asia-Pacific are improving affordability and accessibility. This supportive ecosystem encourages healthcare providers to integrate liquid biopsy into standard diagnostic pathways, reducing barriers for patients and strengthening clinical acceptance globally.
Growing awareness of early detection is enhancing acceptance across patient and clinician groups. National cancer screening initiatives highlight the importance of liquid biopsy in cost-efficient and patient-friendly diagnostics. The integration of liquid biopsy into healthcare workflows reflects the rising demand for non-invasive solutions that reduce patient discomfort while delivering high clinical accuracy. As awareness expands, liquid biopsy is expected to gain broader traction across oncology and non-oncology applications, sustaining market growth in the coming decade.
Key Takeaways
- The liquid biopsy market was valued at USD 10.3 billion in 2023 and is projected to reach USD 35.3 billion by 2033.
- The market is forecasted to expand at a compound annual growth rate (CAGR) of 13.1% during the period from 2023 to 2033.
- Multi-Gene-Parallel Analysis (NGS) held a significant share of 72.8% of the global liquid biopsy market revenue in 2023.
- Circular nucleic acids contributed over 34.78% to the total global liquid biopsy revenue in 2023, highlighting their rising clinical importance.
- Instruments dominated the product landscape, accounting for 44.9% of the total market share in the year 2023.
- More than 77% of the liquid biopsy market revenue in 2023 was generated through cancer detection applications.
- Hospitals and laboratories emerged as key end users, holding over 47.2% of the market share in 2023.
- North America led globally in 2023, generating more than 47.1% of total revenues in the liquid biopsy market.
Regional Analysis
North America accounted for the largest revenue share in 2023, holding over 47.1% of the global market. The United States dominated this region, driven by higher investments and the presence of numerous biotechnology companies producing liquid biopsy tests. Support from organizations such as the American Society of Clinical Oncology (ASCO) further encouraged adoption. These factors are expected to drive continued market expansion in North America. Strong awareness, technological advances, and supportive regulatory frameworks are fueling demand for advanced diagnostic solutions.
Canada is gradually following the United States in adopting liquid biopsy testing, with regulations allowing only FDA-approved tests. Several biotechnology companies are actively competing in the market, which is expected to contribute to regional growth over the forecast period. Government initiatives are also playing a major role. Increased subsidies and investments directed toward the development of liquid biopsy technologies are projected to strengthen the market outlook. This is creating opportunities for both domestic players and international firms entering Canada.
A significant development in Canada was BioMark Diagnostics Solutions Inc. securing US$825,000 in funding for its lung cancer screening method. The funding was largely provided by the Consortium for Industrial Research and Innovation in Medical Technology, Spark Grant from the Canadian Cancer Society, and Brain Canada Foundation. These initiatives highlight increasing support for innovative solutions in cancer diagnostics. Such funding activities are expected to accelerate research and commercialization efforts. This reflects the region’s strong commitment to promoting advanced, non-invasive diagnostic technologies.
Segmentation Analysis
The Multi-Gene-Parallel Analysis (NGS) segment dominated the liquid biopsy technology market in 2023, contributing over 72.8% of global revenues. NGS enables the detection of mutations driving tumorigenesis and resistance mechanisms developed after treatment. Advances in NGS have significantly reduced sequencing costs while enhancing accuracy and precision. This technology facilitates the detection of unknown variants and ctDNA mutations with high sensitivity. NGS has proven effective in detecting early-stage cancers, including stage 1 and 2 lung cancer, with a mutation allele frequency as low as 0.1%.
The market has been driven forward by continuous technological innovation. Twist Bioscience Corp. introduced the Twist NGS Methylation Detection System in 2021, focusing on liquid biopsy and epigenetic analysis. Similarly, Biodesix Inc. launched a 52-gene NGS blood test to expand molecular testing capabilities. These advancements reflect industry leaders’ commitment to enhancing diagnostic outcomes. Growing adoption of such high-precision NGS systems has contributed to strengthening the technology segment and is expected to further propel market penetration in the coming years.
In terms of biomarkers, Circular Nucleic Acids held the largest share, accounting for 34.78% of global revenues in 2023. This category has demonstrated wide clinical utility in liquid biopsy applications, particularly in analyzing circulating tumor DNA (ctDNA). CtDNA plays a critical role in diagnostic laboratories and treatment monitoring by offering real-time insights into tumor progression. It has become a valuable tool for prognosis and targeted therapy decisions. Its capacity to serve as a non-invasive alternative to traditional biopsy methods is fostering rapid adoption across oncology diagnostics.
Academic and research centers are increasingly utilizing ctDNA-based liquid biopsies in translational cancer studies. Their use has provided new opportunities to expand applications in early detection, treatment monitoring, and cancer recurrence evaluation. CtDNA profiling allows molecular-level insights that improve patient-specific therapies. With continuous clinical validation, ctDNA biomarker analysis is expected to broaden its adoption across multiple cancer types. This segment remains pivotal in advancing precision medicine, offering a safer and more efficient substitute to invasive diagnostic procedures in oncology.
From a product perspective, instruments represented the largest market share at 44.9% in 2023. These devices enable accurate collection and analysis of liquid biopsy samples, including circulating tumor cells (CTCs) and ctDNA. Increasing adoption of automated, advanced instruments in hospitals and laboratories has bolstered their market growth. Consumables such as kits and reagents followed, showing strong growth due to rising test volumes. Additionally, software and services contributed significantly by facilitating accurate interpretation of results, further highlighting the importance of digital integration within this sector.
In terms of applications, cancer remained the leading domain, representing more than 77% of the market in 2023. Liquid biopsy has been highly effective for diagnosis and monitoring across multiple cancers such as lung, prostate, breast, colorectal, leukemia, and gastrointestinal. Beyond oncology, liquid biopsy is gaining traction in reproductive health for non-invasive prenatal testing and fertility screening. On the end-use side, hospitals and laboratories accounted for 47.2% of the market share due to their advanced infrastructure and skilled workforce. Specialty clinics and research institutions also played important roles in advancing this technology’s clinical adoption.
Key Players Analysis
The liquid biopsy market is expanding as leading players enhance their cancer diagnostics portfolios. Their focus on product diversification is strengthening market growth prospects. At the same time, the industry is experiencing progress in non-cancer applications, which is broadening clinical relevance. This trend is encouraging higher adoption of liquid biopsy technologies across healthcare systems. The introduction of advanced solutions is enabling early disease detection, improving patient management, and supporting the ongoing transformation of diagnostic practices worldwide.
Key players in the competitive landscape are pursuing strategies centered on product innovation. They are launching new liquid biopsy solutions to support advanced clinical applications and extend their market reach. These efforts are aligned with the rising demand for minimally invasive diagnostic methods. The emphasis on research, development, and approvals is shaping a more competitive environment. Industry experts anticipate that such developments will accelerate adoption and contribute significantly to the improvement of healthcare infrastructure globally.
For instance, F. Hoffmann-La Roche AG has established a prominent presence in the global liquid biopsy market. In August 2020, the company received approval from the U.S. Food and Drug Administration for its FoundationOne Liquid CDx test. This diagnostic solution is designed for the detection of solid tumors, reinforcing Roche’s leadership in precision oncology. The approval not only strengthened the company’s cancer diagnostics portfolio but also highlighted the importance of regulatory support in advancing innovative products that enhance patient outcomes and clinical decision-making.
FAQ
1. What is liquid biopsy?
Liquid biopsy is a modern diagnostic method used to detect cancer in a non-invasive way. It works by analyzing tumor-derived materials such as circulating tumor DNA, tumor cells, or exosomes found in blood and other body fluids. Unlike tissue biopsy, it requires only a simple sample, often blood. This makes the process safer, quicker, and less painful. Doctors use it to study genetic changes, monitor treatment, and track cancer progression without frequent invasive procedures.
2. How does liquid biopsy work?
Liquid biopsy works by detecting fragments of tumor material that are released into body fluids like blood or urine. These fragments may include circulating tumor DNA (ctDNA), tumor cells, or proteins. Advanced methods such as next-generation sequencing and PCR help in analyzing these biomarkers. The test provides information about genetic mutations or cancer-related changes in real time. This allows doctors to detect cancer earlier, choose targeted therapies, and monitor treatment response, offering a less invasive alternative to tissue biopsies.
3. What are the main applications of liquid biopsy?
Liquid biopsy has several important applications in cancer care. It is widely used for early cancer detection and for guiding therapy selection. Doctors also use it to monitor treatment effectiveness and check for minimal residual disease after therapy. It helps in identifying cancer recurrence and tracking metastasis across different organs. Its non-invasive nature allows repeated testing over time. This makes it valuable for personalized medicine, where treatment is adjusted according to ongoing tumor activity and patient response.
4. Which cancers can be detected by liquid biopsy?
Liquid biopsy can be applied to multiple types of cancer. It is most commonly used in lung, breast, colorectal, prostate, and pancreatic cancers. These cancers release detectable levels of tumor DNA or cells into the bloodstream. Studies show promising results for its use in ovarian and brain cancers as well. Research is ongoing to expand its scope into hematological cancers. Its growing accuracy and adoption suggest that liquid biopsy will soon cover a wider range of tumor types globally.
5. What are the advantages of liquid biopsy over traditional biopsy?
Liquid biopsy offers several advantages compared to conventional tissue biopsy. It is safer, quicker, and non-invasive, requiring only blood or fluid samples. Patients face less discomfort and reduced risk of complications. It provides real-time insights into tumor changes and allows doctors to monitor treatment response effectively. Another advantage is its ability to capture tumor heterogeneity, which tissue biopsy often misses. Liquid biopsy also supports frequent testing, making it a powerful tool for ongoing cancer management and personalized treatment.
6. What are the limitations of liquid biopsy?
Despite its benefits, liquid biopsy has limitations. It may have lower sensitivity in detecting cancers at very early stages, especially when tumor DNA is scarce. In some cases, results may not fully replace information gained from tissue biopsy. High testing costs and limited insurance coverage are also challenges in certain regions. Standardization of methods across labs is still lacking. Regulatory approval processes vary globally, slowing adoption. While effective, it is often used alongside, not as a replacement for, tissue biopsy.
7. What biomarkers are commonly analyzed in liquid biopsy?
The most common biomarkers analyzed in liquid biopsy include circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), exosomes, and microRNAs. These biomarkers provide valuable insights into the genetic and molecular changes in tumors. ctDNA is widely studied for detecting mutations linked to cancer growth. CTCs help in understanding tumor spread and progression. Exosomes and microRNAs carry proteins and genetic material that reveal tumor activity. Analyzing these biomarkers gives doctors real-time data that aids in diagnosis, treatment decisions, and monitoring outcomes.
8. How large is the global liquid biopsy market?
The global liquid biopsy market is expanding rapidly. The global liquid biopsy market size is expected to be worth around USD 35.3 billion by 2033, from USD 10.3 billion in 2023, growing at a CAGR of 13.1% during the forecast period from 2023 to 2033. This rise is driven by the increasing cancer burden worldwide, greater adoption of personalized medicine, and advancements in sequencing technologies. The market is expected to play a key role in cancer screening and management. High investment in research is also pushing liquid biopsy into mainstream oncology diagnostics.
9. What factors are driving market growth?
Several factors are driving the growth of the liquid biopsy market. The rising global prevalence of cancer is one of the strongest drivers. The need for safer, non-invasive diagnostic procedures is also increasing. Advances in next-generation sequencing and PCR are improving test accuracy and efficiency. Precision medicine is expanding, and liquid biopsy supports this shift by guiding therapy selection. Growing healthcare spending and research investments are accelerating adoption. Together, these factors are creating strong demand for liquid biopsy worldwide.
10. Which regions dominate the liquid biopsy market?
North America dominates the global liquid biopsy market, driven by advanced healthcare infrastructure and strong research activities. Favorable reimbursement policies also support market expansion in this region. Europe holds a significant share due to active cancer screening programs and government healthcare initiatives. The Asia-Pacific region is the fastest growing, with rising cancer cases, government investments, and better diagnostic access. Countries such as China and India are investing heavily in modern healthcare, making Asia a key growth engine.
11. Who are the key players in the liquid biopsy market?
The liquid biopsy market is led by several major players. Key companies include ANGLE plc, Oncimmune, Guardant Health Inc., Myriad Genetics Inc., Biocept Inc., Lucence Health Inc., Freenome Holdings Inc., F. Hoffmann-La Roche Ltd., QIAGEN, Illumina Inc., Thermo Fisher Scientific Inc., Epigenomics AG, and Other Key Players. These companies are investing heavily in research, mergers, and collaborations to expand their offerings. Startups and biotech firms are also entering the market with innovative solutions. Their combined efforts are driving rapid advancements in test accuracy and availability. Intense competition among these players is accelerating market growth and expanding access to liquid biopsy technologies globally.
12. Which segment holds the largest share of the liquid biopsy market?
Circulating tumor DNA (ctDNA) is the largest biomarker segment in the liquid biopsy market. It provides detailed insights into cancer mutations, making it highly valuable for therapy selection. Among applications, therapy monitoring and recurrence detection are leading due to high demand in oncology practice. Lung cancer contributes the largest share among cancer types, as it is one of the most diagnosed cancers globally. This focus is expected to continue, with growing research expanding into other cancer segments as well.
13. What are the challenges faced by the market?
The liquid biopsy market faces several challenges despite its growth. High testing costs limit patient access in many countries. Sensitivity issues, particularly in early-stage cancers, affect reliability. Standardization of testing protocols across laboratories is lacking, which can lead to variations in results. Regulatory approval processes are complex and slow, delaying widespread adoption. Limited awareness among healthcare providers in some regions is another barrier. Overcoming these challenges will be crucial to ensure broader clinical use of liquid biopsy worldwide.
14. What are the future trends in the liquid biopsy market?
Future trends indicate significant advancements in the liquid biopsy market. Artificial intelligence will enhance data interpretation, improving test accuracy. Multi-cancer detection tests are gaining momentum, enabling simultaneous screening for several cancers. The use of liquid biopsy for monitoring immunotherapy responses is expanding. Partnerships between biotech firms and healthcare providers are increasing to accelerate adoption. As costs decline and access improves, liquid biopsy will become a standard tool in oncology. These trends suggest strong growth and wider adoption globally.
Conclusion
The global liquid biopsy market is set for strong growth, driven by rising cancer cases and the need for safer, non-invasive diagnostic methods. Liquid biopsy is becoming an important tool for early cancer detection, treatment monitoring, and precision medicine. Advancements in sequencing and molecular diagnostics have improved accuracy, making it more effective across oncology and beyond, including prenatal testing and transplant monitoring. Supportive regulations, growing awareness, and increased funding are accelerating adoption worldwide. With its ability to provide real-time insights and reduce reliance on invasive procedures, liquid biopsy is positioned to play a central role in shaping the future of diagnostics across multiple healthcare fields.
View More
Liquid Biopsy Market || Breast Biopsy Device Market || Cancer Biopsy Market || Biopsy Needle Market || Biopsy Device Market
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



