Introduction
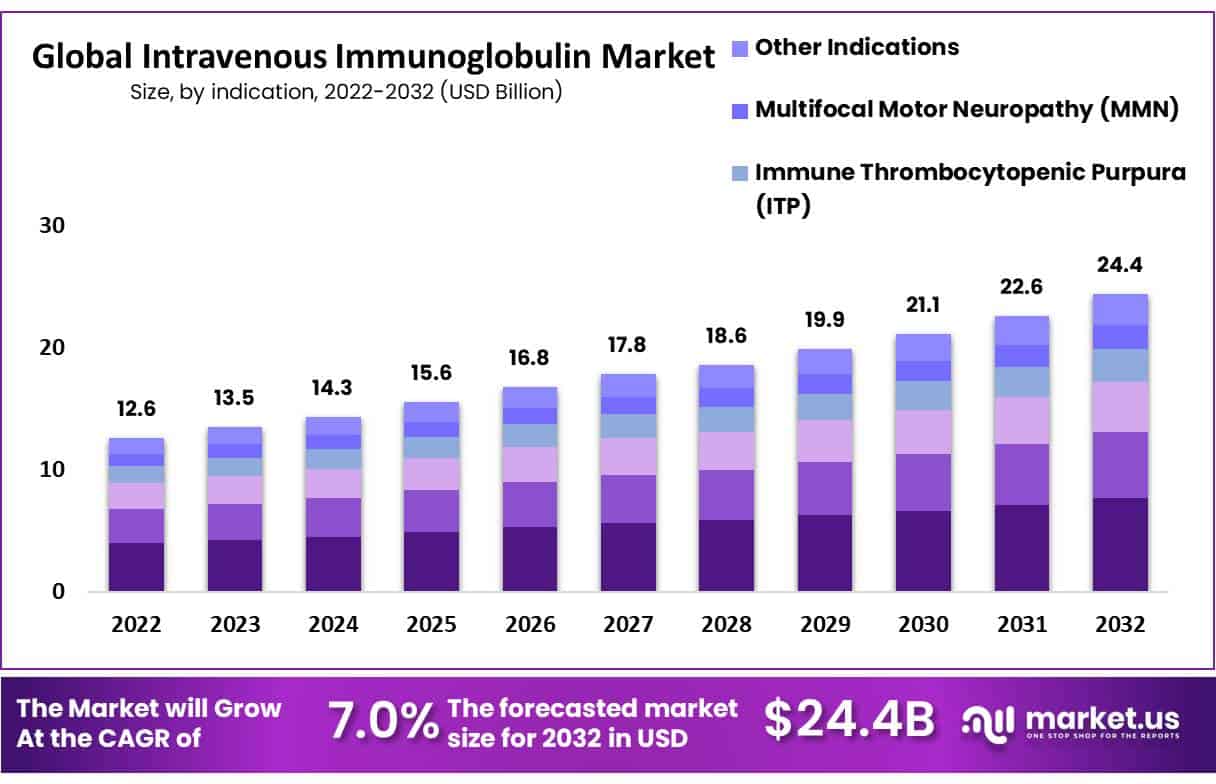
The Intravenous Immunoglobulin (IVIG) market, valued at USD 13.6 billion in 2022, is projected to grow to approximately USD 24.4 billion by 2032, with a CAGR of 7.0% from 2023 to 2032. This growth is primarily driven by the increasing adoption of IVIG therapies for various autoimmune and inflammatory diseases. The market expansion is further supported by the FDA’s approval of new IVIG treatments, such as those for dermatomyositis, demonstrating the widening scope of therapeutic applications.
Significant changes in healthcare policies also influence the IVIG market’s trajectory. The integration of home IVIG infusions into Medicare as a permanent benefit from January 2024 reflects a shift towards more accessible treatment options. This change follows a demonstration project that confirmed the effectiveness and patient preference for home-based treatments over traditional outpatient settings.
To address challenges like supply shortages, the IVIG market is adopting more efficient dosing strategies tailored to individual patient needs. Innovations such as dosing based on adjusted body weight (ABW) and ideal body weight (IBW) are optimizing therapy effectiveness and minimizing wastage. These approaches are supported by growing pharmacokinetic data that highlight their economic and therapeutic benefits.
Furthermore, advancements in administration methods are enhancing patient convenience. An increasing number of patients now receive IVIG through subcutaneous methods, which are less invasive and maintain consistent IgG levels in the blood. This method allows for home administration, reducing clinical overheads and improving patient quality of life.
Key Takeaways
- The Intravenous Immunoglobulin (IVIG) Market was valued at USD 12.6 billion in 2022 and is projected to reach USD 24.4 billion by 2032.
- The market is anticipated to grow at a compound annual growth rate (CAGR) of 7.0% between 2023 and 2032, showing steady expansion.
- North America accounted for 49.5% of the global market share in 2022, dominating the intravenous immunoglobulin industry by region.
- Over 500,000 Americans are diagnosed with at least 200 primary immunodeficiency disorders (PIDDs), significantly contributing to the demand for IVIG therapies.
- The cost of intravenous immunoglobulin is USD 73.89 per kilogram, with treatment expenses varying based on individual disease severity.
- IVIG therapy costs can reach up to USD 10,000 for severe cases, making it a costly but essential treatment option.
- Long-term replacement therapy using immunoglobulin G (IgG) can exceed USD 30,000 annually for patients with chronic immune conditions.
- Primary Immunodeficiency accounted for 31.6% of the total market share in 2022, leading among the key application segments for IVIG.
- Hospital pharmacies generated over 57.3% of the revenue in the IVIG market in 2022, reflecting their pivotal role in product distribution.
- On average, IVIG therapy involves 12 to 16 sessions annually, depending on the patient’s condition and severity of the disease.
- Grifols S.A. completed the acquisition of Biotest on April 25, 2022, strengthening its position in the IVIG market.
- Among 100 individuals with symptomatic immune diseases, 70% were infected with SARS-CoV-2, underlining a heightened vulnerability among this group.
- Of those infected, 69% required hospitalization, and 8% succumbed to the complications caused by SARS-CoV-2 infections.
Regional Analysis
North America led the market in 2022, holding a significant 49.5% share. This dominance is attributed to increased awareness about immunodeficiency disorder treatments, growing interest among clinicians, and rising healthcare spending. Intravenous immunoglobulin (IVIG) treatments have gained traction due to advances in healthcare infrastructure and rapid U.S. FDA approvals. The market is poised for growth, driven by these factors and upcoming product approvals from global organizations like the WHO. These developments are enhancing the region’s position as a key player in the immunoglobulin market.
The industry is expanding due to changing lifestyles and rising chronic illnesses among older adults. Autoimmune diseases, a major market driver, are the third leading cause of chronic illness in the U.S. Forecasts suggest strong growth due to increasing primary immunodeficiency (PID) cases. By 2050, NIOSH estimates 5% to 8% of Americans may be affected by autoimmune diseases. These conditions are becoming more frequent for unknown reasons, further accelerating market demand. This trend underlines the urgent need for advanced treatment options and improved healthcare solutions.
The Asia Pacific region is expected to experience significant market growth during the forecast period. Factors contributing to this growth include increased awareness of immunoglobulin-based therapies and an aging population. Emerging economies and rising healthcare spending also play a vital role in this expansion. Improved healthcare facilities and rising cases of immune disorders are boosting the adoption of immunoglobulin therapies. The rapidly growing market in this region reflects its potential to become a key contributor to the global immunoglobulin industry in the near future.
The market outlook for immunoglobulin therapies remains promising globally. Advances in treatment options and increased approval rates from international health bodies are driving growth. North America’s dominance is complemented by Asia Pacific’s rising demand, fueled by healthcare improvements and increasing awareness. Autoimmune diseases remain a key driver, with a growing patient base demanding effective solutions. With enhanced healthcare infrastructure, an aging population, and a strong focus on addressing immune deficiencies, the global immunoglobulin market is expected to sustain robust growth in the coming years.
Emerging Trends
- Expanded Clinical Applications: IVIG therapy is now applied beyond primary immunodeficiency disorders. It is increasingly used to treat Kawasaki disease, autoimmune conditions, and specific neuropathies. This shift highlights the growing versatility of IVIG in managing complex medical conditions. Physicians recognize its role in addressing inflammatory processes and immune modulation. These applications are supported by clinical trials and emerging data. As a result, IVIG’s therapeutic scope continues to broaden, providing critical treatment options for patients who have limited alternatives. This expanded usage underscores the need for robust supply chains to meet growing demand effectively.
- Dosing Strategies in Obese Patients: Recent studies explore IVIG dosing strategies in obese patients. Adjusted body weight-based dosing has been investigated for its efficacy. Findings indicate that antibody level increases are smaller in obese individuals compared to those with normal BMI. However, current evidence does not strongly recommend altering dosing solely based on BMI. This area requires further research to validate optimal dosing strategies for different body types. Adapting dosing for specific populations could improve outcomes while ensuring cost-effective and efficient IVIG usage across healthcare settings.
- Supply Challenges: The United States has faced IVIG shortages since late 2018. This issue worsened during the COVID-19 pandemic due to increased demand. These challenges have prompted discussions about optimizing dosing to conserve supply. Ensuring availability requires efficient manufacturing processes and equitable distribution systems. Healthcare providers are also exploring alternative therapies to address shortages. Long-term solutions include increasing production capacity and improving global supply chains. Addressing these challenges is critical to maintaining patient access to IVIG therapy for life-saving treatments.
Use Cases
- Kawasaki Disease: IVIG is a primary treatment for Kawasaki disease, a condition causing blood vessel inflammation. It effectively reduces the risk of coronary artery aneurysms to less than 5%. Around 80% of patients respond well to this therapy. It is typically administered in high doses within the first 10 days of illness. Early treatment is crucial to minimize long-term cardiovascular complications. IVIG also helps reduce fever and other inflammatory symptoms. This makes it a key intervention in managing this potentially serious condition.
- Primary Immunodeficiency Diseases (PIDDs): IVIG is vital for patients with Primary Immunodeficiency Diseases (PIDDs). It replaces missing antibodies, strengthening the immune system and preventing infections. The dosage depends on the patient’s total body weight, with adjustments made for obese individuals. IVIG is usually administered every 3-4 weeks to maintain adequate antibody levels. It significantly improves the quality of life by reducing hospitalizations and severe infections. For many, it is a lifelong treatment essential for managing their condition.
- Autoimmune Neuropathies: IVIG is widely used to treat autoimmune neuropathies like multifocal motor neuropathy. It helps improve muscle strength and reduces disability caused by the condition. The treatment’s duration and dosage depend on the patient’s symptoms and response. Regular IVIG infusions are often required to maintain benefits. This therapy is particularly effective in slowing disease progression and improving mobility. It has become a cornerstone in managing these complex neurological disorders.
- Immune Thrombocytopenic Purpura (ITP): IVIG is a key treatment for Immune Thrombocytopenic Purpura (ITP). It rapidly increases platelet counts, reducing the risk of severe bleeding. The standard dose is 1 gram per kilogram of body weight, given over one to two days. IVIG is often used during acute bleeding episodes or pre-surgery to stabilize platelet levels. It is a highly effective short-term solution, particularly for patients who do not respond to other treatments. Regular monitoring ensures optimal outcomes in managing this blood disorder.
Conclusion
The Intravenous Immunoglobulin (IVIG) market is poised for steady growth, driven by rising demand for advanced treatments for autoimmune and immune-related disorders. Expanding clinical applications, improved administration methods, and innovative dosing strategies are enhancing patient care and therapy efficiency. Challenges like supply shortages are being addressed through optimized manufacturing and distribution. The market benefits from increased awareness, healthcare advancements, and supportive policy changes, such as home infusion coverage. With a growing patient base and regional opportunities in Asia Pacific and North America, the IVIG market holds significant potential. Continued innovation and investment will be key to meeting the rising demand for these life-saving therapies, ensuring better outcomes for patients globally.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



