Table of Contents
Overview
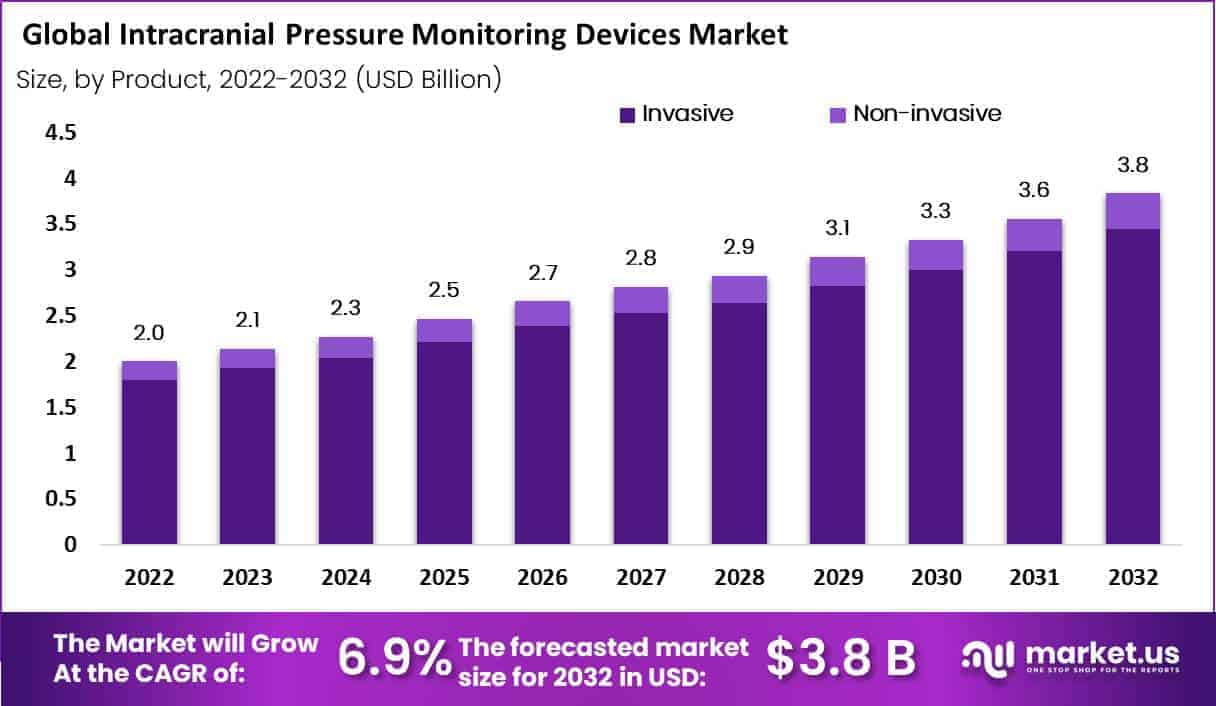
New York, NY – June 23, 2025 – In 2022, the global Intracranial Pressure Monitoring Devices Market accounted for USD 2.0 Billion and will reach USD 3.8 Billion by 2032. Between 2023 and 2032, this market is estimated to register a CAGR of 6.9%.
The global Intracranial Pressure (ICP) Monitoring Devices Market is poised for robust expansion over the next decade, driven by a rising incidence of traumatic brain injuries (TBI), stroke, and other neurological conditions. The market is expected to experience steady growth, supported by increasing demand for early detection technologies and advancements in minimally invasive monitoring solutions.
ICP monitoring devices are used to measure the pressure inside the skull, particularly in patients with severe head trauma, hydrocephalus, or post-neurosurgical complications. These devices help healthcare providers detect elevated intracranial pressure in real time, which is critical for timely intervention and improved patient outcomes.
Growing awareness of brain injury management, improved neurosurgical care infrastructure, and rising adoption of critical care equipment in emerging markets are key factors contributing to market expansion. In addition, the development of implantable and wireless ICP monitors is reshaping clinical protocols by offering continuous monitoring with reduced infection risks.
North America is anticipated to remain the leading region due to high healthcare expenditure, while Asia-Pacific is projected to witness the fastest growth, owing to a rising patient pool and expanding hospital infrastructure. The ongoing push for improved neurological diagnostics is expected to accelerate innovation and investment in ICP monitoring technologies through 2033.
Key Takeaways
- Clinical Importance: Intracranial pressure (ICP) monitoring is essential for diagnosing and managing patients with brain injuries, intracranial hemorrhages, hydrocephalus, or other neurological conditions. It helps prevent brain damage by providing accurate, continuous tracking of ICP fluctuations.
- Device Options: A wide range of ICP monitoring devices is available, including external ventricular drains (EVDs), intraparenchymal monitors, subdural sensors, epidural sensors, and noninvasive alternatives. Selection depends on clinical requirements and patient-specific conditions.
- Traumatic Brain Injuries: ICP monitoring is crucial for managing traumatic brain injuries, especially those resulting from accidents. These devices assist in detecting and treating elevated pressure levels that may become life-threatening if left unchecked.
- Neurosurgery and Neurology: ICP monitoring devices are routinely used in neurosurgical and neurological settings to deliver real-time pressure data. This information supports clinical decision-making regarding treatments, surgical procedures, or other interventions.
- Emergency Medicine: In emergency care, ICP monitoring is used to assess patients with head trauma. It helps medical teams determine the severity of injury and guides timely, appropriate treatment strategies.
- Neurocritical Care: Neurocritical care units incorporate ICP monitoring as a core component of care for severe neurological conditions. Continuous monitoring allows for prompt therapeutic responses and ongoing evaluation of intracranial dynamics.
Segmentation Analysis
- By Product Analysis: In 2022, the invasive segment held the largest share in the intracranial pressure (ICP) monitoring devices market and is expected to contribute 90% of total revenue during the forecast period. Invasive ICP monitoring involves the direct insertion of a catheter or sensor into brain tissue or cerebrospinal fluid, offering high accuracy. Despite its precision, this method carries risks such as infection or complications due to its invasive nature but remains the clinical gold standard.
- By Application Analysis: The traumatic brain injury (TBI) segment dominates the application category, accounting for 34% of the intracranial pressure monitoring devices market. TBI frequently leads to elevated intracranial pressure, a critical complication that requires continuous monitoring. ICP devices help clinicians make timely treatment decisions, minimize secondary brain injury, and improve outcomes. As TBI cases rise due to accidents and trauma, the demand for monitoring in this segment remains consistently strong.
- By End-User Analysis: Hospitals and clinics represent the leading end-user segment, holding 78% of the revenue share in the ICP monitoring devices market. These facilities provide advanced neurocritical care and conduct most ICP monitoring procedures, especially for trauma and neurosurgery patients. Their specialized infrastructure, availability of skilled personnel, and integration of intensive care protocols contribute to their significant market dominance throughout the forecast period.
Market Segments
Based on Product
- Invasive
- Microtransducer ICP Monitoring
- External Ventricular Drainage (EVD)
- Non-invasive
- Transcranial Doppler Ultrasonography
- Tympanic Membrane Displacement (TMD)
- Optic Nerve Sheath Diameter
- MRI/CT
- Fundoscopy (papilledema)
Based on Application
- Traumatic Brain Injury
- Intracerebral Hemorrhage
- Meningitis
- Subarachnoid Hemorrhage
- CSF Management
- Migraine
- Stroke
- Hydrocephalus
- EEG
- Other Applications
Based on End-User
- Hospitals and Clinic
- Trauma Centers
Regional Analysis
- North America Analysis: North America is projected to maintain its dominance in the global intracranial pressure monitoring devices market. This leadership is supported by the high prevalence of traumatic brain injuries, well-established healthcare infrastructure, the presence of key market players, and favorable reimbursement frameworks. The United States represents the largest market within the region, while Canada also plays a substantial role in supporting regional growth through expanded adoption and clinical use of ICP monitoring technologies.
- Asia Pacific Analysis: Asia Pacific is anticipated to be the fastest-growing region in the intracranial pressure monitoring devices market during the forecast period. This growth is driven by a rising incidence of traumatic brain injuries, an expanding elderly population, and continuous improvements in healthcare infrastructure. China and India are the primary contributors to this growth, with additional momentum coming from developed healthcare systems in Japan, South Korea, and Australia.
Emerging Trends
- Shift toward non-invasive, headband-based sensors: A major trend is the replacement of intraparenchymal catheters with non-invasive headband sensors. In March 2024, the U.S. FDA cleared the B4C System (Addition of BcSs-PICNIW-2000 Sensor), which uses strain-gauge sensors on a headband to capture skull deformations and generate surrogate ICP waveforms for clinical interpretation. This approach reduces infection risk and allows preliminary ICP assessment without surgical placement.
- Integration of machine learning for waveform analysis: Recent research has demonstrated that artificial intelligence can enhance the accuracy of non-invasive ICP estimates. A study published in npj Digital Medicine (February 2025) developed a machine-learning model that analyzes waveform parameters from the B4C cranial extensometer device, achieving improved mean ICP estimation compared to earlier algorithms. This trend supports the use of predictive analytics to refine non-invasive measurements and guide timely interventions.
- Emergence of fully implantable, wireless telemetry systems: Long-term, continuous ICP monitoring outside the ICU is now feasible through wireless implantable sensors. The FDA approved the AURA™ ICP Monitoring System (Branchpoint Technologies) as the first fully implantable wireless ICP sensor, enabling mobile monitoring of pressure dynamics in ambulatory settings. Clinicians can obtain real-time data without externalized catheters, expanding ICP tracking into outpatient and rehabilitation environments.
- Global prioritization and expanded access initiatives: The World Health Organization’s 2021 List of Priority Medical Devices for management of neurological emergencies explicitly includes ICP monitoring equipment, underscoring its essential role in critical-care settings. This designation is driving efforts to standardize device availability and training in low-resource regions. In turn, manufacturers and health systems are collaborating to ensure ICP monitors meet robustness and ease-of-use criteria for broader deployment.
Use Cases
- Neurocritical Care in Severe Traumatic Brain Injury: In patients with Glasgow Coma Scale scores < 8 or abnormal head CT findings, intraventricular catheters remain the gold standard for guiding ICP-targeted therapy. ICP monitoring initiates when pressures exceed 20–25 mm Hg, helping to prevent cerebral ischemia and herniation. Guidelines recommend ICP monitoring in TBI cases meeting two or more criteria—age > 40 years, hypotension (SBP < 90 mm Hg), or motor posturing to optimize intervention timing and reduce mortality.
- Management of Hydrocephalus and Shunt Surveillance: Telemetric ICP sensors implanted within cerebrospinal fluid shunts enable continuous assessment of shunt function without repeated hospital visits. Early detection of shunt malfunction often signaled by sustained pressure elevations—can reduce emergency revisions. Studies report catheter misplacement rates > 30% in freehand ventriculostomy, underscoring the value of guided, telemetric monitoring for accurate pressure trends and timely intervention.
- Intraoperative Monitoring during Neurosurgical Procedures: During craniotomies and decompressive surgeries, parenchymal fiber-optic probes provide immediate feedback on ICP fluctuations. These probes allow real-time waveform analysis, including detection of Lundberg A and B waves, enabling surgeons to adjust decompression strategies and maintain cerebral perfusion pressure. Complication rates for ventricular catheters include hemorrhage (10 %), infection (20 %), and technical misplacement (5 %), emphasizing the importance of precise monitoring.
- Bedside Screening for Intracranial Hypertension: In settings where invasive monitoring is contraindicated, optic nerve sheath diameter ultrasound offers a rapid, bedside screen for elevated ICP. Though its ± 10–15 mm Hg error margin limits precise titration, this non-invasive method assists in triaging patients with suspected idiopathic intracranial hypertension or mild head trauma, facilitating timely transfer to neurocritical units for definitive monitoring and care.
Conclusion
The global intracranial pressure (ICP) monitoring devices market is set to witness sustained growth through 2033, driven by rising neurological disorders, advances in minimally invasive technologies, and increasing demand for early intervention tools. Invasive methods remain the clinical gold standard, while non invasive and wireless innovations are expanding accessibility and reducing risks.
North America leads in adoption due to strong infrastructure, while Asia-Pacific shows the fastest growth trajectory. Emerging trends such as AI integration and WHO prioritization are shaping the market landscape. ICP monitoring continues to be vital in critical care, neurosurgery, and emergency medicine, reinforcing its clinical and commercial relevance.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



