Table of Contents
Introduction:
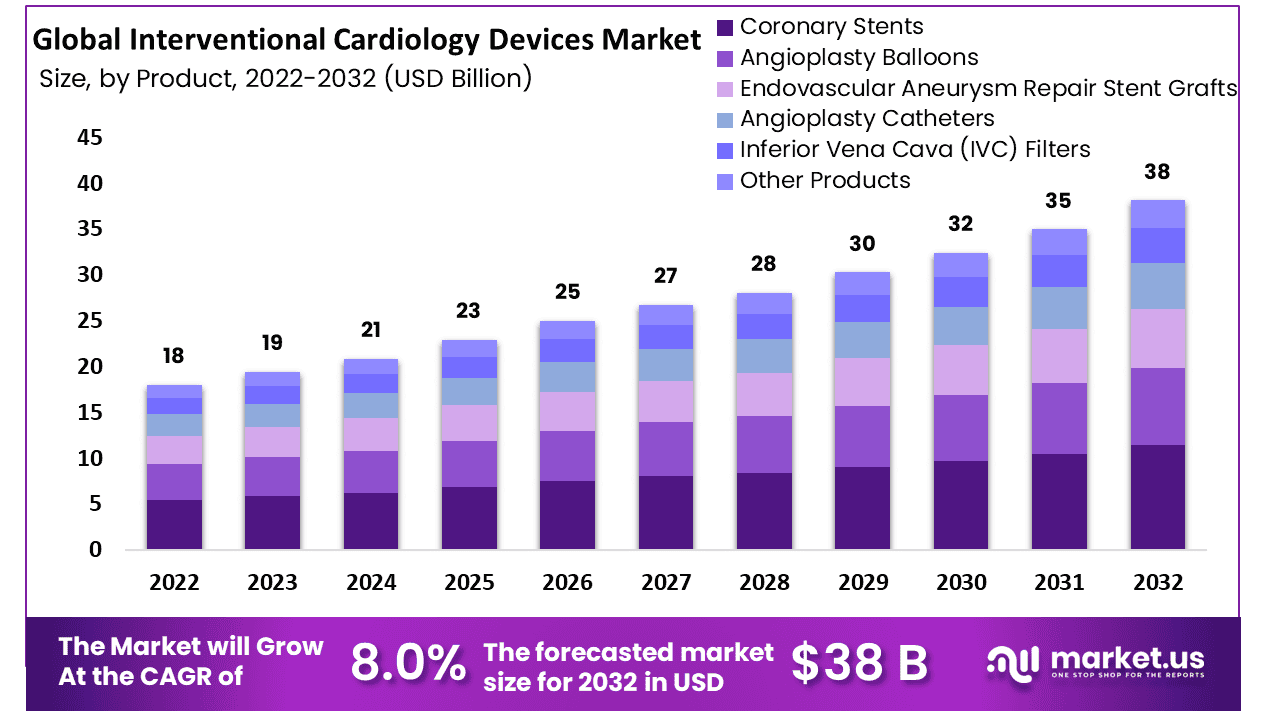
The Global Interventional Cardiology Devices Market size is expected to be worth around USD 38 Billion by 2032 from USD 19 Billion in 2023, growing at a CAGR of 8.0% during the forecast period from 2024 to 2032.
The Interventional Cardiology Devices Market is witnessing robust growth, driven by the rising prevalence of cardiovascular diseases and technological advancements in medical devices. According to the National Institutes of Health (NIH), cardiovascular diseases remain the leading cause of death globally, necessitating innovative and effective treatment solutions. Interventional cardiology procedures, which include the use of devices such as stents, catheters, and balloons, offer minimally invasive alternatives to traditional surgery, reducing recovery time and improving patient outcomes. The increasing incidence of lifestyle-related conditions like obesity and diabetes is further fueling the demand for interventional cardiology devices.
Technological advancements play a significant role in the growth of the interventional cardiology market. Innovations such as drug-eluting stents and bioresorbable vascular scaffolds are enhancing the efficacy and safety of interventional procedures. The FDA highlights the importance of regulatory science research to ensure these devices meet safety standards and improve patient outcomes. The integration of imaging technologies with interventional devices is also improving the precision and effectiveness of procedures, leading to better patient care. Moreover, the shift towards personalized medicine is encouraging the development of customized interventional cardiology solutions.
Despite the promising growth, the market faces several challenges. High costs associated with advanced interventional devices can limit their accessibility, particularly in low-income regions. There is also a need for skilled healthcare professionals to perform complex interventional procedures, which may pose a barrier in areas with limited resources. Regulatory hurdles and the need for continuous innovation to address emerging healthcare needs further complicate the market landscape. Efforts by leading institutions like the NIH and FDA to advance research and streamline approval processes are essential to overcoming these challenges and supporting market growth.
Recent developments in the interventional cardiology market include strategic collaborations and significant investments in research and development by leading medical device companies. These efforts aim to introduce advanced technologies and expand the range of available interventional treatments. For example, ongoing trials and studies focus on improving device safety and efficacy, which is crucial for gaining regulatory approvals and increasing market adoption. The growing emphasis on developing minimally invasive procedures and enhancing patient outcomes continues to drive innovation and growth in the interventional cardiology devices market.
Key Takeaways
- Market Size: The Global Interventional Cardiology Devices Market size is expected to be worth around USD 38 Billion by 2032 from USD 19 Billion in 2023.
- Market Growth: The Global Interventional Cardiology Devices Market is growing at a CAGR of 8.0% during the forecast period from 2024 to 2032.
- Product Analysis: Coronary stents had the highest revenue share more than 35.0%.
- End-User Analysis: The Hospital sector dominates the market with the largest market share of 54.6%.
- Regional Analysis: The market for interventional cardiology devices is dominated by North America.
- Technological Advancements: Innovations in devices like drug-eluting stents, bioresorbable vascular scaffolds, and advanced imaging technologies are enhancing procedural safety and efficacy. These advancements improve patient outcomes by providing more precise and effective treatments.
- Minimally Invasive Procedures: Interventional cardiology offers minimally invasive alternatives to traditional surgeries, reducing recovery times and hospital stays. This makes procedures more appealing to patients and healthcare providers.
- Regulatory Support: Efforts by institutions like the FDA to streamline approval processes and advance regulatory science research are crucial for market growth, ensuring that new devices meet safety and efficacy standards.
- Challenges: High costs and the need for skilled professionals can limit accessibility, especially in low-income regions. Regulatory hurdles and continuous innovation demands are ongoing challenges.
- Personalized Medicine: The shift towards personalized treatment plans is driving the development of customized interventional devices, tailored to meet individual patient needs.
Interventional Cardiology Devices Statistics
- Coronary Stents: Over 1 million coronary stents are implanted annually in the U.S., making them one of the most common interventional cardiology devices.
- Angioplasty Procedures: Around 500,000 angioplasty procedures are performed yearly in the U.S., highlighting the widespread use of interventional cardiology techniques.
- Drug-Eluting Stents: Approximately 90% of stents used in interventional procedures are drug-eluting stents, which help prevent re-narrowing of arteries.
- Peripheral Intervention Devices: There were around 400,000 peripheral vascular interventions in the U.S. in 2023, with the number growing due to aging populations.
- Transcatheter Aortic Valve Replacement (TAVR): TAVR procedures increased by 15% annually in the U.S., with around 80,000 procedures performed each year.
- Ventricular Assist Devices (VADs): Over 4,000 VADs are implanted annually in the U.S., primarily in patients awaiting heart transplants.
- Chronic Total Occlusion (CTO) Procedures: CTO procedures make up about 15-20% of all percutaneous coronary interventions (PCI) in the U.S.
- Cardiac Ablation Devices: The number of cardiac ablation procedures in the U.S. exceeded 100,000 annually, primarily used to treat atrial fibrillation.
- Balloon Angioplasty: Approximately 200,000 balloon angioplasties are performed annually in the U.S., often used in conjunction with stent placements.
- Carotid Artery Stenting: Around 25,000 carotid artery stenting procedures are performed each year in the U.S., aimed at preventing strokes.
- Intracoronary Imaging: The use of intracoronary imaging devices, such as IVUS and OCT, has seen a 10% increase in adoption per year for improving diagnostic accuracy.
- Left Atrial Appendage Closure (LAAC): There are approximately 30,000 LAAC procedures annually in the U.S., a growing area of interventional cardiology
Interventional Cardiology Devices Segments
Based on Product
- Angioplasty Balloons
- Coronary Stents
- Angioplasty Catheters
- Endovascular Aneurysm Repair Stent Grafts
- Inferior Vena Cava (IVC) Filters
- Plaque Modification Devices
- Hemodynamic Flow Alteration Devices
- Other Products
Based on End-User
- Hospitals
- Ambulatory Surgical Centers
- Cardiac Catheterization Laboratories
- Other End-users
Emerging Trends
- Integration of Artificial Intelligence: AI is increasingly being used to guide interventional procedures, such as cardiac ultrasound imaging, to improve accuracy and reduce errors in diagnostics and treatment planning.
- Development of Low-Field MRI Systems: New low-field MRI technologies are being developed to improve compatibility with interventional devices, enhancing image-guided procedures by reducing the risk of heating and making imaging more affordable.
- Advancements in Catheter Technologies: Innovations in catheter-based treatments are expanding the range of conditions treatable with minimally invasive methods, including structural heart diseases.
- Focus on Hemodynamics: Research is being conducted on non-clinical test methods to assess device-patient interactions under simulated conditions, aiming to improve the safety and effectiveness of cardiovascular devices.
- Use of Bioresorbable Stents: There is a growing trend towards using bioresorbable stents that naturally dissolve in the body over time, reducing the need for long-term medication to prevent restenosis.
- Increased Device Durability: Efforts are being made to improve the mechanical durability of cardiovascular implants through better preclinical testing and material science.
- Personalized Interventions: Devices are being developed with customization options to better fit individual patient anatomies, enhancing treatment outcomes.
- Remote Monitoring Capabilities: Many interventional devices are now equipped with technologies that allow for remote monitoring of patients, improving follow-up care and management.
Use Cases
- Percutaneous Coronary Intervention (PCI): Interventional cardiology devices like stents and balloons are widely used in PCI to open blocked coronary arteries, improving blood flow to the heart. This procedure is crucial for treating acute coronary syndromes and chronic coronary artery disease.
- Drug-Eluting Stents: These stents are coated with medication that helps prevent restenosis, or the re-narrowing of arteries, after PCI. They are a key advancement in reducing the need for repeat procedures.
- Transcatheter Aortic Valve Replacement (TAVR): TAVR is a minimally invasive procedure using catheter-based devices to replace damaged aortic valves, especially in patients who are at high surgical risk.
- Balloon Angioplasty: This technique involves using a balloon-tipped catheter to widen narrowed or obstructed arteries or veins, a fundamental procedure in managing coronary artery disease.
- Intravascular Imaging: Devices like intravascular ultrasound (IVUS) and optical coherence tomography (OCT) provide detailed images of blood vessels, aiding in the precise placement of stents and assessment of artery blockages.
- Atherectomy Devices: These devices remove plaque from blood vessels, improving blood flow and reducing the risk of complications during angioplasty. They are particularly useful for treating heavily calcified lesions.
- Embolic Protection Devices: These devices are used during procedures like carotid artery stenting to capture and remove debris, preventing it from traveling to the brain and causing strokes.
- Left Atrial Appendage Closure: Devices used for closing the left atrial appendage in patients with atrial fibrillation help reduce the risk of stroke by preventing blood clots from forming.
Conclusion
The Interventional Cardiology Devices Market is experiencing significant growth, driven by the rising prevalence of cardiovascular diseases and advancements in medical technology. Key innovations such as drug-eluting stents and bioresorbable scaffolds are improving patient outcomes, while the shift towards minimally invasive procedures is increasing demand. However, challenges such as high costs, regulatory hurdles, and the need for skilled professionals persist. Strategic collaborations and investments in research are essential for overcoming these barriers. The market is expected to reach USD 38 billion by 2032, with North America leading in adoption, particularly within hospital settings.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



