Table of Contents
Introduction
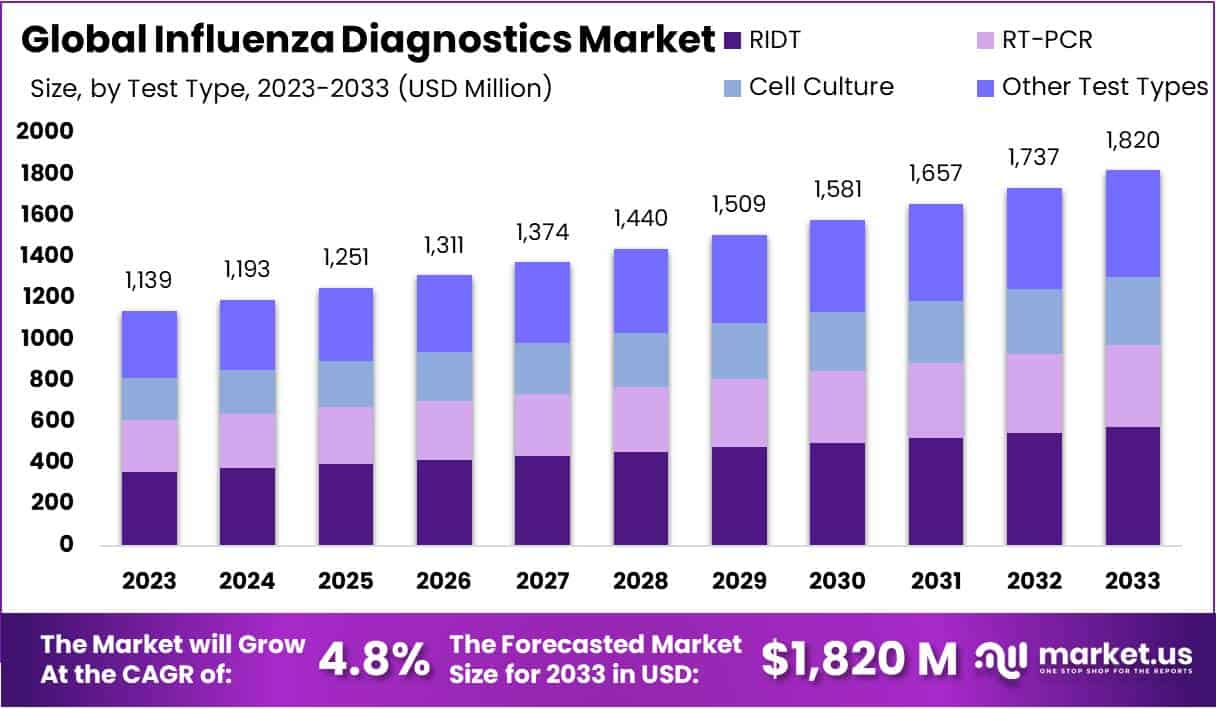
The Influenza Diagnostics Market is expected to grow significantly, reaching approximately USD 1,820 million by 2033 from USD 1,139 million in 2023. This expansion is driven by a 4.8% Compound Annual Growth Rate (CAGR) from 2024 to 2033. The market’s growth is influenced by technological advancements, increased pandemic preparedness, and the rising demand for point-of-care (POC) testing. Enhanced diagnostic capabilities are improving influenza detection, leading to greater adoption in clinical settings.
One of the key drivers of market expansion is the advancement of molecular diagnostic technologies. Nucleic acid amplification tests (NAATs) and other modern methods provide higher sensitivity and specificity than traditional approaches. These improvements enhance the accuracy of influenza detection, reducing false negatives and enabling timely treatment decisions. The demand for rapid and precise diagnostic solutions is increasing, particularly in hospitals and laboratories.
Another major factor contributing to market growth is the emphasis on point-of-care (POC) testing. Rapid influenza diagnostic tests (RIDTs) have gained popularity due to their ability to deliver results within minutes. The speed and convenience of RIDTs make them essential tools for healthcare providers, especially during seasonal flu outbreaks. The integration of micro- and nanotechnology in diagnostic platforms has further enhanced the efficiency and accessibility of these tests.
The market is also benefiting from global pandemic preparedness efforts. The impact of past influenza pandemics has highlighted the importance of early detection and containment. Governments and healthcare organizations are increasing investments in diagnostic infrastructure and research. This has led to the development of more efficient and scalable testing solutions. The expansion of diagnostic facilities, particularly in emerging economies, is expected to further support market growth.
In conclusion, the Influenza Diagnostics Market is set for steady growth, driven by technological advancements, rising adoption of POC testing, and increased pandemic preparedness. Innovations in molecular diagnostics and microtechnologies are improving test sensitivity and speed, making them more accessible. With continuous research and investment in diagnostic solutions, the market is expected to witness sustained demand, ensuring better influenza management worldwide.
Key Takeaways
- Market Growth Outlook: The influenza diagnostics market is projected to reach USD 1820 million by 2033, expanding at a 4.8% CAGR from 2024 to 2033.
- Recent Investments: Major funding, including Abbott Laboratories’ USD 314 million, highlights strong financial backing for cutting-edge diagnostic innovations and enhanced testing solutions.
- Innovative Market Trends: Companies like Roche Diagnostics, LumiraDx, and BGI Genomics drive advancements in user-friendly and high-precision influenza testing technologies.
- Strategic Industry Alliances: Key partnerships, such as BD’s acquisition of BioGX and Qiagen’s collaboration with Alnylam Pharmaceuticals, are reshaping the competitive landscape.
- Dominant Test Type in 2023: Rapid Influenza Diagnostic Tests (RIDTs) led the market with a 31.6% share, owing to their fast turnaround time and operational efficiency.
- Growth in Molecular Diagnostics: PCR-based molecular diagnostics are gaining traction, offering superior sensitivity and specificity, enhancing early influenza detection.
- Top End-Use Segment: Hospitals dominated the market in 2023, holding a 43.4% share, due to integrated diagnostic and treatment services.
- Regional Market Leader: North America led with a 41.2% market share in 2023, fueled by advanced healthcare infrastructure and strong investments.
- Fastest-Growing Region: Asia-Pacific emerges as the fastest-growing market, driven by rising healthcare spending and increasing influenza prevalence.
- Technology-Driven Opportunities: Advances in molecular diagnostics, AI integration, and point-of-care testing create new opportunities for market growth and better accessibility.
Emerging Trends
- Growth of Rapid Diagnostic Tests (RDTs): Rapid Influenza Diagnostic Tests (RIDTs) are now essential tools for detecting influenza A and B. These tests deliver results in just 10–15 minutes. Their high specificity ensures fewer false positives. However, sensitivity levels range between 50% and 70%. This variation means molecular assays are often needed for confirmation. Despite this limitation, RIDTs remain popular for their speed and ease of use. Researchers continue to improve their accuracy, making them a critical part of influenza diagnostics.
- Rise of Point-of-Care Testing (POCT): Point-of-Care Testing (POCT) is changing the way healthcare providers detect influenza. These tests provide immediate results, helping doctors make quick decisions. Faster diagnosis means early antiviral treatment, improving patient outcomes. POCT also reduces hospital overcrowding by preventing unnecessary admissions. Studies show that timely treatment lowers healthcare costs. As technology advances, POCT devices are becoming more affordable and user-friendly. This trend ensures that accurate influenza testing is accessible in hospitals, clinics, and even pharmacies.
- Advancements in Molecular Assays: Nucleic Acid Amplification Tests (NAATs) are now the gold standard for detecting influenza. These tests, including RT-PCR, provide unmatched accuracy. They detect even low levels of the virus, improving early diagnosis. Unlike traditional methods, NAATs offer both high sensitivity and specificity. This accuracy helps identify different influenza strains, aiding in outbreak management. Researchers are also working on making these tests faster and more portable. With ongoing advancements, molecular assays will continue to improve global influenza surveillance and control.
- Wastewater Surveillance for Early Detection: Monitoring wastewater is an emerging strategy in influenza surveillance. This method helps detect influenza viruses before widespread outbreaks occur. Wastewater testing can identify viral particles weeks in advance. This early warning system gives public health officials time to prepare. By tracking trends, authorities can predict infection waves and take preventive measures. Wastewater surveillance is already used for COVID-19 tracking. Its success suggests that it could become a standard tool for influenza monitoring.
- The Rise of At-Home Combination Testing: At-home diagnostic tests are becoming more common. New combination tests can detect both influenza and COVID-19. These tests allow people to identify infections quickly without visiting a doctor. Early detection helps individuals take appropriate measures, such as self-isolation or seeking medical advice. The convenience of at-home testing reduces the spread of infections. As consumer demand grows, companies are improving the accuracy and affordability of these tests. The future of influenza diagnostics includes more reliable, easy-to-use home testing solutions.
Use Cases
- Clinical Diagnosis and Patient Management: Timely and accurate flu testing helps doctors distinguish influenza from other respiratory infections. Early diagnosis allows for prompt antiviral treatment, reducing symptoms and complications. This is crucial for high-risk individuals, such as the elderly and those with chronic illnesses. Without proper testing, flu symptoms can be mistaken for other diseases, delaying treatment. Rapid testing in clinics and hospitals improves patient outcomes and helps prevent severe cases.
- Hospital Infection Control: Hospitals use flu diagnostics to detect cases early and prevent outbreaks. Rapid identification of infected patients allows for quick isolation, reducing the risk of spreading the virus to others. This is especially important in crowded hospital settings, where influenza can spread rapidly. Effective infection control protects both patients and healthcare workers. By using flu tests in emergency rooms and intensive care units, hospitals can ensure better containment of infections.
- Public Health Surveillance: Flu diagnostics help track virus activity in communities. Public health agencies use testing data to monitor outbreaks and identify seasonal trends. This information guides vaccination campaigns and other preventive strategies. By analyzing flu test results, health officials can predict the severity of upcoming flu seasons. Early detection of outbreaks allows for timely interventions, such as recommending vaccinations or promoting hygiene measures. Surveillance efforts improve flu preparedness on a national and global scale.
- Detection of Zoonotic Transmission: Some flu strains originate from animals, such as birds and pigs. Detecting these strains early is essential for preventing pandemics. Hospitals now test severe flu cases for avian influenza, including H5N1. This approach helps identify zoonotic transmission before it spreads widely. Early detection allows public health authorities to take action, such as monitoring infected individuals and controlling animal outbreaks. Improved flu diagnostics strengthen global pandemic preparedness and reduce the risk of widespread transmission.
- At-Home Testing for Early Intervention: At-home flu tests allow individuals to check for infection without visiting a doctor. FDA-approved tests can detect both influenza and COVID-19. Early detection helps people seek medical advice quickly and start treatment sooner. This reduces the risk of severe symptoms and complications. At-home tests also lower the burden on hospitals during peak flu seasons. By identifying flu cases early, individuals can take precautions to prevent spreading the virus to others.
Conclusion
The influenza diagnostics market is set for steady growth, driven by advancements in molecular testing, point-of-care solutions, and pandemic preparedness efforts. Rapid and accurate detection methods are improving patient outcomes by enabling early treatment and reducing the spread of infections. Increased investment in diagnostic research and infrastructure is further supporting market expansion. The demand for convenient and fast testing solutions, including at-home kits, is also rising. Emerging technologies, such as AI-driven diagnostics and wastewater surveillance, are enhancing influenza monitoring. With continuous innovation and global health initiatives, the market is expected to remain strong, providing better access to effective flu detection tools and improving overall disease management worldwide.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



